Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma
- PMID: 33132633
- PMCID: PMC7579760
- DOI: 10.3748/wjg.v26.i38.5759
Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma
Abstract
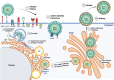
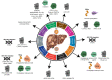
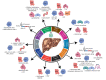
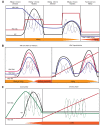
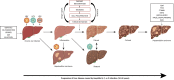
Chronic infection with viral hepatitis affects half a billion individuals worldwide and can lead to cirrhosis, cancer, and liver failure. Liver cancer is the third leading cause of cancer-associated mortality, of which hepatocellular carcinoma (HCC) represents 90% of all primary liver cancers. Solid tumors like HCC are complex and have heterogeneous tumor genomic profiles contributing to complexity in diagnosis and management. Chronic infection with hepatitis B virus (HBV), hepatitis delta virus (HDV), and hepatitis C virus (HCV) are the greatest etiological risk factors for HCC. Due to the significant role of chronic viral infection in HCC development, it is important to investigate direct (viral associated) and indirect (immune-associated) mechanisms involved in the pathogenesis of HCC. Common mechanisms used by HBV, HCV, and HDV that drive hepatocarcinogenesis include persistent liver inflammation with an impaired antiviral immune response, immune and viral protein-mediated oxidative stress, and deregulation of cellular signaling pathways by viral proteins. DNA integration to promote genome instability is a feature of HBV infection, and metabolic reprogramming leading to steatosis is driven by HCV infection. The current review aims to provide a brief overview of HBV, HCV and HDV molecular biology, and highlight specific viral-associated oncogenic mechanisms and common molecular pathways deregulated in HCC, and current as well as emerging treatments for HCC.
Keywords: Chronic viral infection; Hallmarks of cancer; Hepatitis B virus; Hepatitis C virus; Hepatitis delta virus co-infection; Hepatocellular carcinoma; Molecular mechanisms; Viral hepatitis.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors have no conflict of interest to declare.
Figures


References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health. 2016;4:e609–e616. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

