Tacrolimus and mycophenolate mofetil as second-line treatment in autoimmune hepatitis: Is the evidence of sufficient quality to develop recommendations?
- PMID: 33132643
- PMCID: PMC7579758
- DOI: 10.3748/wjg.v26.i38.5896
Tacrolimus and mycophenolate mofetil as second-line treatment in autoimmune hepatitis: Is the evidence of sufficient quality to develop recommendations?
Abstract
Background: The standard management of autoimmune hepatitis (AIH) is based on corticosteroids, alone or in combination with azathioprine. Second-line treatments are needed for patients who have refractory disease. However, high-quality data on the alternative management of AIH are scarce.
Aim: To evaluate the efficacy and safety of tacrolimus and mycophenolate mofetil (MMF) and the quality of evidence by using the Grading of Recommendations Assessment, Development and Evaluation approach (GRADE).
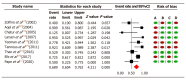
Methods: A systematic review and meta-analysis of the available data were performed. We calculated pooled event rates for three outcome measures: Biochemical remission, adverse events, and mortality, with their corresponding 95% confidence intervals (CI).
Results: The pooled biochemical remission rate was 68.9% (95%CI: 60.4-76.2) for tacrolimus, and 59.6% (95%CI: 54.8-64.2) for MMF, and rates of adverse events were 25.5% (95%CI: 12.4-45.3) for tacrolimus and 24.1% (95%CI: 15.4-35.7) for MMF. The pooled mortality rate was estimated at 11.5% (95%CI: 7.1-18.1) for tacrolimus and 9.01% (95%CI: 6.2-12.8) for MMF. Pooled biochemical remission rates for tacrolimus and MMF in patients with intolerance to standard therapy were 56.6% (CI: 43.4-56.6) vs 73.5% (CI: 58.1-84.7), and among non-responders were 59.1% (CI: 48.7-68.8) vs 40.8% (CI: 32.3-50.0), respectively. Moreover, the overall quality assessments using GRADE proved to be very low for all our outcomes in both treatment groups.
Conclusion: Tacrolimus and MMF are in practice considered effective for patients with AIH who are non-responders or intolerant to first-line treatment, but we found no high-quality evidence to support this statement.
Keywords: Autoimmune hepatitis; Efficacy; Grading of Recommendations Assessment, Development and Evaluation approach; Meta-analysis; Second-line; Systematic review.
©The Author(s) 2020. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Figures



References
-
- Ngu JH, Gearry RB, Frampton CM, Stedman CA. Mortality and the risk of malignancy in autoimmune liver diseases: a population-based study in Canterbury, New Zealand. Hepatology. 2012;55:522–529. - PubMed
-
- Hoeroldt B, McFarlane E, Dube A, Basumani P, Karajeh M, Campbell MJ, Gleeson D. Long-term outcomes of patients with autoimmune hepatitis managed at a nontransplant center. Gastroenterology. 2011;140:1980–1989. - PubMed
-
- Liberal R, Krawitt EL, Vierling JM, Manns MP, Mieli-Vergani G, Vergani D. Cutting edge issues in autoimmune hepatitis. J Autoimmun. 2016;75:6–19. - PubMed
-
- Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54–66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

