Hepatoprotective properties of red betel (Piper crocatum Ruiz and Pav) leaves extract towards H2O2-induced HepG2 cells via anti-inflammatory, antinecrotic, antioxidant potency
- PMID: 33132711
- PMCID: PMC7584795
- DOI: 10.1016/j.jsps.2020.08.007
Hepatoprotective properties of red betel (Piper crocatum Ruiz and Pav) leaves extract towards H2O2-induced HepG2 cells via anti-inflammatory, antinecrotic, antioxidant potency
Abstract
Background: Prolonged exposure of free radicals, or known as reactive oxygen species (ROS), in hepatic cells may cause oxidative stress. Without proper treatment, it can induce liver injury and fatal hepatic disease, including cirrhosis. Red betel (Piper crocatum Ruiz and Pav) is one of Indonesia's medicinal plants that has been known to exhibit antioxidant, anti-inflammatory activities. This study aims to determine hepatoprotective effect of red betel leaves extract (RBLE) towards liver injury.
Method: Hydrogen peroxide-induced HepG2 cells were used as liver injury model·H2O2-induced HepG2 cells were treated with 25 µg/mL and 100 µg/mL RBLE. Several parameters were observed, including TNF-α level through ELISA; necrotic, apoptotic, dead, live cells; and ROS level through flow cytometry analysis; and GPX gene expression through qPCR.
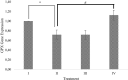
Result: The study showed that treatment with RBLE were able to decrease TNF-α level; necrotic and death cells percentage; as well as ROS level. On the other hand, it were able to increase apoptotic and live cells percentage; as well as GPX gene expression. Low concentration (25 µg/mL) of RBLE treatment exhibited stronger anti-inflammatory activity as it was resulted in the lower TNF-α level and were able to switched hepatic cell death pathway from necrosis to apoptosis as shown by the shifted of apoptotic cells and necrotic cells percentage. This lead to lower death cells and ultimately improve live cells percentage. Meanwhile high concentration of RBLE (100 µg/mL) exhibited stronger antioxidant properties as indicated by lower ROS level and higher GPX gene expression.
Conclusion: Overall, this study was able to demonstrate hepatoprotective effect of RBLE towards liver injury model through its anti-inflammatory and antioxidant activities.
Keywords: Anti-inflammatory; Antinecrotic; Antioxidant; HepG2; Hepatoprotective; Red betel.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Hepatotoxicity prevention in Acetaminophen-induced HepG2 cells by red betel (Piper crocatum Ruiz and Pav) extract from Indonesia via antioxidant, anti-inflammatory, and anti-necrotic.Heliyon. 2021 Jan 4;7(1):e05620. doi: 10.1016/j.heliyon.2020.e05620. eCollection 2021 Jan. Heliyon. 2021. PMID: 33474504 Free PMC article.
-
Phytochemical Profile of Antibacterial Agents from Red Betel Leaf (Piper crocatum Ruiz and Pav) against Bacteria in Dental Caries.Molecules. 2022 Apr 30;27(9):2861. doi: 10.3390/molecules27092861. Molecules. 2022. PMID: 35566225 Free PMC article. Review.
-
Anti-allergic Inflammatory Components from the Leaves of Piper crocatum Ruiz & Pav.Biol Pharm Bull. 2021;44(2):245-250. doi: 10.1248/bpb.b20-00726. Biol Pharm Bull. 2021. PMID: 33518676
-
Hepatoprotective potency of Litsea glutinosa (L.) C.B. Rob. leaf methanol extract on H2O2-induced toxicity in HepG2 cells.J Ethnopharmacol. 2023 Mar 25;304:116076. doi: 10.1016/j.jep.2022.116076. Epub 2022 Dec 22. J Ethnopharmacol. 2023. PMID: 36567040
-
Anti-Infection of Oral Microorganisms from Herbal Medicine of Piper crocatum Ruiz & Pav.Drug Des Devel Ther. 2024 Jun 25;18:2531-2553. doi: 10.2147/DDDT.S453375. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38952486 Free PMC article. Review.
Cited by
-
Photoprotective Agents Obtained from Aromatic Plants Grown in Colombia: Total Phenolic Content, Antioxidant Activity, and Assessment of Cytotoxic Potential in Cancer Cell Lines of Cymbopogon flexuosus L. and Tagetes lucida Cav. Essential Oils.Plants (Basel). 2022 Jun 27;11(13):1693. doi: 10.3390/plants11131693. Plants (Basel). 2022. PMID: 35807645 Free PMC article.
-
Antidiabetic and hepatoprotection effect of butterfly pea flower (Clitoria ternatea L.) through antioxidant, anti-inflammatory, lower LDH, ACP, AST, and ALT on diabetes mellitus and dyslipidemia rat.Heliyon. 2024 Apr 16;10(8):e29812. doi: 10.1016/j.heliyon.2024.e29812. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38681657 Free PMC article.
-
Blood Glucose Level, Langerhans Pancreas and Lipid Profile of Diabetic Rats After Administration of Red Betel, Ginger and Cinnamon Combination Extract.Trop Life Sci Res. 2023 Mar;34(1):41-50. doi: 10.21315/tlsr2023.34.1.3. Epub 2023 Mar 31. Trop Life Sci Res. 2023. PMID: 37065797 Free PMC article.
-
Hepatoprotective effects of Curcuma xanthorrhiza Roxb. extract via free radical scavenger, inhibiting apoptosis and inflammation mechanisms in acetaminophen-induced liver injury.Iran J Basic Med Sci. 2025;28(8):1100-1106. doi: 10.22038/ijbms.2025.82500.17833. Iran J Basic Med Sci. 2025. PMID: 40584441 Free PMC article.
-
Mechanism of the effect of Piper crocatum extract on wound healing of Wistar rats post-excision mammary tumor based on IL-10 level, TGF-β1 expression, VEGF expression, Collagen density, and clinical features.Open Vet J. 2025 Mar;15(3):1264-1278. doi: 10.5455/OVJ.2025.v15.i3.18. Epub 2025 Mar 31. Open Vet J. 2025. PMID: 40276181 Free PMC article.
References
-
- Afifah E., Mozef T., Sandra F., Arumwardana S., Rihibiha D.D., Nufus H., Rizal R., Amalia A., Bachtiar I., Murti H., Widowati W. Induction of matrix metalloproteinases in chondrocytes by interleukin IL-1β as an osteoarthritis model. J. Math. Fundam. Sci. 2019;51(2):103–111.
-
- Anugrahwati M., Purwaningsih T., Manggalarini J.A., Alnavis N.B., Wulandari D.N., Pranowo H.D. Extraction of ethanolic extract of red betel leaves and its cytotoxicity test on HeLa cells. Procedia Eng. 2016;148:1402–1407.
LinkOut - more resources
Full Text Sources

