Differences in Breast Muscle Mitochondrial Respiratory Capacity, Reactive Oxygen Species Generation, and Complex Characteristics between 7-week-old Meat- and Laying-type Chickens
- PMID: 33132733
- PMCID: PMC7596037
- DOI: 10.2141/jpsa.0190133
Differences in Breast Muscle Mitochondrial Respiratory Capacity, Reactive Oxygen Species Generation, and Complex Characteristics between 7-week-old Meat- and Laying-type Chickens
Abstract
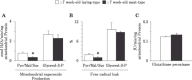
The skeletal muscle growth rate is a major feature differentiating meat- and laying-type chickens. A large amount of ATP is required during skeletal muscle synthesis, in which mitochondrial energy production capacities play a significant role. Additionally, mitochondria may participate in muscle protein degradation via reactive oxygen species generation. To investigate the differences in mitochondrial energetic characteristics between chickens exhibiting different growth rates, this study evaluated respiratory capacities in response to different types of respiratory substrate, protein abundances, assembly of individual respiratory complexes (I-V) and supercomplexes, and reactive oxygen species generation rates. These characteristics were compared between mitochondria from the breast muscle (M. pectoralis superficialis) of seven-week-old meat- and laying-type male chickens. Blue native polyacrylamide gel electrophoresis analysis revealed that meat-type chickens exhibited a significantly lower protein abundance of complex III (cytochrome bc 1 complex), complex V (F0F1 ATP synthase), and total amount of supercomplexes than did laying-type chickens. There were no differences between chicken types in the respiration rate of mitochondria incubated with either pyruvate/malate or succinate, each of which drives complex I- and complex II-linked respiration. Carnitine palmitoyltransferase-1-dependent and -independent respiration during ATP synthesis and carnitine palmitoyltransferase-2 enzymatic activity were significantly lower in meat-type chickens than in layingtype chickens. For mitochondria receiving pyruvate/malate plus succinate, the reactive oxygen species generation rate and its ratio to the oxygen consumed (the percentage of free radical leak) were also significantly lower in meat-type chickens than in laying-type chickens. These results suggested that the mitochondrial energetic capacities of the breast muscle of meat-type chickens could be lower than those of laying-type chickens at seven weeks of age. Furthermore, the lower reactive oxygen species generation rate in meat-type chickens might have implications for rapid muscle development, which is possibly related to their lower muscle protein degradation rates.
Keywords: cardiolipin; carnitine palmitoyltransferase system; fatty acid oxidation; reactive oxygen species production; respiratory complex; respiratory supercomplex.
2020, Japan Poultry Science Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Acín-Pérez R, Fernández-Silva P, Peleato ML, Pérez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Molecular Cell, 32: 529-539. 2008. - PubMed
-
- Barceló-Coblijn G, Murphy EJ. An improved method for separating cardiolipin by HPLC. Lipids, 43: 971-976. 2008. - PubMed
-
- Dudkina NV, Kouril R, Peters K, Braun HP, Boekema EJ. Structure and function of mitochondrial supercomplexes. Biochimica et Biophysica Acta, 1797: 664-670. 2010. - PubMed
-
- Estabrook RW. Mitochondrial respiratory control and the polarographic measurement of ADP:O ratios. Methods Enzymol, 10: 41-47. 1967
-
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. Journal of Biological Chemistry, 226: 497-509. 1957. - PubMed

