Effects of Akt/mTOR/p70S6K Signaling Pathway Regulation on Neuron Remodeling Caused by Translocation Repair
- PMID: 33132828
- PMCID: PMC7550644
- DOI: 10.3389/fnins.2020.565870
Effects of Akt/mTOR/p70S6K Signaling Pathway Regulation on Neuron Remodeling Caused by Translocation Repair
Abstract
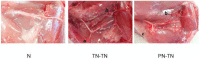
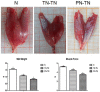
Peripheral nerve injury repair has been considered a difficult problem in the field of trauma for a long time. Conventional surgical methods are not applicable in some special types of nerve injury, prompting scholars to seek to develop more effective nerve translocation repair technologies. The purpose of this study was to explore the functional state of neurons in injured lower limbs after translocation repair, with a view to preliminarily clarify the molecular mechanisms underlying this process. Eighteen Sprague-Dawley rats were divided into the normal, tibial nerve in situ repair, and common peroneal nerve transposition repair tibial nerve groups. Nerve function assessment and immunohistochemical staining of neurofilament 200 (NF-200), protein kinase B (Akt), mammalian target of rapamycin (mTOR), and ribosomal protein S6 kinase (p70S6K) in the dorsal root ganglia were performed at 12 weeks after surgery. Tibial nerve function and neuroelectrophysiological analysis, osmic acid staining, muscle strength testing, and muscle fiber staining showed that the nerve translocation repair could restore the function of the recipient nerve to a certain extent; however, the repair was not as efficient as the in situ repair. Immunohistochemical staining showed that the translocation repair resulted in changes in the microstructure of neuronal cell bodies, and the expressions of Akt, mTOR, and p70S6K in the three dorsal root ganglia groups were significantly different (p < 0.05). This study demonstrates that the nerve translocation repair technology sets up a new reflex loop, with the corresponding neuroskeletal adjustments, in which, donor neurons dominate the recipient nerves. This indicates that nerve translocation repair technology can lead to neuronal remodeling and is important as a supplementary treatment for a peripheral nerve injury. Furthermore, the Akt/mTOR/p70S6K signaling pathway may be involved in the formation of the new neural reflex loop created as a result of the translocation repair.
Keywords: dorsal root ganglion; peripheral nerve injury; remodeling; signaling pathway; translocation repair.
Copyright © 2020 Yuan, Li, Yu, Kang, Xu and Zhang.
Figures






Similar articles
-
The Exploratory Study of the PTEN-AKT/mTOR Signaling Pathway in the Corresponding Dorsal Root Ganglion during Compensatory Repair via Small Gap Amplification in Sciatic Nerve Injury.J Integr Neurosci. 2024 Aug 21;23(8):157. doi: 10.31083/j.jin2308157. J Integr Neurosci. 2024. PMID: 39207068
-
ATP-mediated protein kinase B Akt/mammalian target of rapamycin mTOR/p70 ribosomal S6 protein p70S6 kinase signaling pathway activation promotes improvement of locomotor function after spinal cord injury in rats.Neuroscience. 2010 Sep 1;169(3):1046-62. doi: 10.1016/j.neuroscience.2010.05.046. Epub 2010 Jun 1. Neuroscience. 2010. PMID: 20678995
-
Reinnervation of spinal cord anterior horn cells after median nerve repair using transposition with other nerves.Neural Regen Res. 2019 Apr;14(4):699-705. doi: 10.4103/1673-5374.247474. Neural Regen Res. 2019. PMID: 30632511 Free PMC article.
-
Repair of long segmental ulnar nerve defects in rats by several different kinds of nerve transposition.Neural Regen Res. 2019 Apr;14(4):692-698. doi: 10.4103/1673-5374.247473. Neural Regen Res. 2019. PMID: 30632510 Free PMC article.
-
Immunohistochemical analysis of the mammalian target of rapamycin signalling pathway in extramammary Paget's disease.Br J Dermatol. 2009 Aug;161(2):357-63. doi: 10.1111/j.1365-2133.2009.09179.x. Epub 2009 Apr 29. Br J Dermatol. 2009. PMID: 19438435
Cited by
-
Salvia plebeia R.Br. and Rosmarinic Acid Attenuate Dexamethasone-Induced Muscle Atrophy in C2C12 Myotubes.Int J Mol Sci. 2023 Jan 18;24(3):1876. doi: 10.3390/ijms24031876. Int J Mol Sci. 2023. PMID: 36768200 Free PMC article.
-
Potential of Lycii Radicis Cortex as an Ameliorative Agent for Skeletal Muscle Atrophy.Pharmaceuticals (Basel). 2024 Apr 4;17(4):462. doi: 10.3390/ph17040462. Pharmaceuticals (Basel). 2024. PMID: 38675422 Free PMC article.
-
Effect of treadmill exercise combined with bone marrow stromal cell transplantation on atrophy-related signaling pathway in the denervated soleus muscle.J Exerc Rehabil. 2021 Dec 27;17(6):395-402. doi: 10.12965/jer.2142618.309. eCollection 2021 Dec. J Exerc Rehabil. 2021. PMID: 35036388 Free PMC article.
-
Chitin Conduits with Different Inner Diameters at Both Ends Combined with Dual Growth Factor Hydrogels Promote Nerve Transposition Repair in Rats.J Funct Biomater. 2023 Aug 28;14(9):442. doi: 10.3390/jfb14090442. J Funct Biomater. 2023. PMID: 37754856 Free PMC article.
-
Mechanism of erythropoietin-induced M2 microglia polarization via Akt / Mtor / P70S6k signaling pathway in the treatment of brain injury in premature mice and its effect on biofilm.Bioengineered. 2022 May;13(5):13021-13032. doi: 10.1080/21655979.2022.2073000. Bioengineered. 2022. PMID: 35611764 Free PMC article.
References
-
- Barbe M. F., Gomez-Amaya S., Braverman A. S., Brown J. M., Lamarre N. S., Massicotte V. S., et al. (2017). Evidence of vagus nerve sprouting to innervate the urinary bladder and clitoris in a canine model of lower motoneuron lesioned bladder. Neurourol. Urodyn. 36 91–97. 10.1002/nau.22904 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

