Chronic Sodium Selenate Treatment Restores Deficits in Cognition and Synaptic Plasticity in a Murine Model of Tauopathy
- PMID: 33132838
- PMCID: PMC7578417
- DOI: 10.3389/fnmol.2020.570223
Chronic Sodium Selenate Treatment Restores Deficits in Cognition and Synaptic Plasticity in a Murine Model of Tauopathy
Abstract
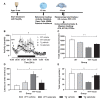
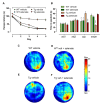
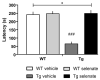
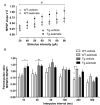
A major goal in diseases is identifying a potential therapeutic agent that is cost-effective and can remedy some, if not all, disease symptoms. In Alzheimer's disease (AD), aggregation of hyperphosphorylated tau protein is one of the neuropathological hallmarks, and Tau pathology correlates better with cognitive impairments in AD patients than amyloid-β load, supporting a key role of tau-related mechanisms. Selenium is a non-metallic trace element that is incorporated in the brain into selenoproteins. Chronic treatment with sodium selenate, a non-toxic selenium compound, was recently reported to rescue behavioral phenotypes in tau mouse models. Here, we focused on the effects of chronic selenate application on synaptic transmission and synaptic plasticity in THY-Tau22 mice, a transgenic animal model of tauopathies. Three months with a supplement of sodium selenate in the drinking water (12 μg/ml) restored not only impaired neurocognitive functions but also rescued long-term depression (LTD), a major form of synaptic plasticity. Furthermore, selenate reduced the inactive demethylated catalytic subunit of protein phosphatase 2A (PP2A) in THY-Tau22 without affecting total PP2A.Our study provides evidence that chronic dietary selenate rescues functional synaptic deficits of tauopathy and identifies activation of PP2A as the putative mechanism.
Keywords: Alzheimer’s disease; chronic oral treatment; long-term depression; neurocognitive functions; protein phosphatase 2A (PP2A); synaptic plasticity; synaptic transmission; tau hyperphosphorylation.
Copyright © 2020 Ahmed, Van der Jeugd, Caillierez, Buée, Blum, D’Hooge and Balschun.
Figures


References
-
- Balschun D., Wolfer D. P., Gass P., Mantamadiotis T., Welzl H., Schutz G., et al. (2003). Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J. Neurosci 23, 6304–6314. 10.1523/JNEUROSCI.23-15-06304.2003 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

