Limitations and Promise of Retinal Tissue From Human Pluripotent Stem Cells for Developing Therapies of Blindness
- PMID: 33132839
- PMCID: PMC7513806
- DOI: 10.3389/fncel.2020.00179
Limitations and Promise of Retinal Tissue From Human Pluripotent Stem Cells for Developing Therapies of Blindness
Abstract
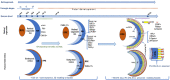
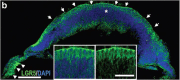
The self-formation of retinal tissue from pluripotent stem cells generated a tremendous promise for developing new therapies of retinal degenerative diseases, which previously seemed unattainable. Together with use of induced pluripotent stem cells or/and CRISPR-based recombineering the retinal organoid technology provided an avenue for developing models of human retinal degenerative diseases "in a dish" for studying the pathology, delineating the mechanisms and also establishing a platform for large-scale drug screening. At the same time, retinal organoids, highly resembling developing human fetal retinal tissue, are viewed as source of multipotential retinal progenitors, young photoreceptors and just the whole retinal tissue, which may be transplanted into the subretinal space with a goal of replacing patient's degenerated retina with a new retinal "patch." Both approaches (transplantation and modeling/drug screening) were projected when Yoshiki Sasai demonstrated the feasibility of deriving mammalian retinal tissue from pluripotent stem cells, and generated a lot of excitement. With further work and testing of both approaches in vitro and in vivo, a major implicit limitation has become apparent pretty quickly: the absence of the uniform layer of Retinal Pigment Epithelium (RPE) cells, which is normally present in mammalian retina, surrounds photoreceptor layer and develops and matures first. The RPE layer polarize into apical and basal sides during development and establish microvilli on the apical side, interacting with photoreceptors, nurturing photoreceptor outer segments and participating in the visual cycle by recycling 11-trans retinal (bleached pigment) back to 11-cis retinal. Retinal organoids, however, either do not have RPE layer or carry patches of RPE mostly on one side, thus directly exposing most photoreceptors in the developing organoids to neural medium. Recreation of the critical retinal niche between the apical RPE and photoreceptors, where many retinal disease mechanisms originate, is so far unattainable, imposes clear limitations on both modeling/drug screening and transplantation approaches and is a focus of investigation in many labs. Here we dissect different retinal degenerative diseases and analyze how and where retinal organoid technology can contribute the most to developing therapies even with a current limitation and absence of long and functional outer segments, supported by RPE.
Keywords: assembloids; disease modeling; drug screening; photoreceptors; pluripotent stem cells; retinal degeneration; retinal organoids; retinal pigment epithelium.
Copyright © 2020 Singh and Nasonkin.
Figures





Similar articles
-
Animal models for the evaluation of retinal stem cell therapies.Prog Retin Eye Res. 2025 May;106:101356. doi: 10.1016/j.preteyeres.2025.101356. Epub 2025 Apr 14. Prog Retin Eye Res. 2025. PMID: 40239758 Free PMC article. Review.
-
Comparison of Developmental Dynamics in Human Fetal Retina and Human Pluripotent Stem Cell-Derived Retinal Tissue.Stem Cells Dev. 2021 Apr;30(8):399-417. doi: 10.1089/scd.2020.0085. Stem Cells Dev. 2021. PMID: 33677999 Free PMC article.
-
Transient Retention of Photoreceptor Outer Segments in Matrigel-Embedded Retinal Organoids.Int J Mol Sci. 2022 Nov 28;23(23):14893. doi: 10.3390/ijms232314893. Int J Mol Sci. 2022. PMID: 36499228 Free PMC article.
-
Looking into the future: Using induced pluripotent stem cells to build two and three dimensional ocular tissue for cell therapy and disease modeling.Brain Res. 2016 May 1;1638(Pt A):2-14. doi: 10.1016/j.brainres.2015.12.011. Epub 2015 Dec 17. Brain Res. 2016. PMID: 26706569 Free PMC article. Review.
-
Decellularised extracellular matrix-derived peptides from neural retina and retinal pigment epithelium enhance the expression of synaptic markers and light responsiveness of human pluripotent stem cell derived retinal organoids.Biomaterials. 2019 Apr;199:63-75. doi: 10.1016/j.biomaterials.2019.01.028. Epub 2019 Jan 22. Biomaterials. 2019. PMID: 30738336
Cited by
-
Organoids for the Study of Retinal Development and Developmental Abnormalities.Front Cell Neurosci. 2021 May 5;15:667880. doi: 10.3389/fncel.2021.667880. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34025363 Free PMC article.
-
Increased IL-6 Levels and the Upregulation of Iron Regulatory Biomarkers Contribute to the Progression of Japanese Encephalitis Virus Infection's Pathogenesis.Neuromolecular Med. 2023 Dec;25(4):596-602. doi: 10.1007/s12017-023-08762-1. Epub 2023 Oct 31. Neuromolecular Med. 2023. PMID: 37907819
-
Microfluidic and Microscale Assays to Examine Regenerative Strategies in the Neuro Retina.Micromachines (Basel). 2020 Dec 9;11(12):1089. doi: 10.3390/mi11121089. Micromachines (Basel). 2020. PMID: 33316971 Free PMC article. Review.
-
Tissue Engineering Strategies for Retina Regeneration.Appl Sci (Basel). 2021 Mar;11(5):2154. doi: 10.3390/app11052154. Epub 2021 Feb 28. Appl Sci (Basel). 2021. PMID: 35251703 Free PMC article.
-
Retinal Organoids: Cultivation, Differentiation, and Transplantation.Front Cell Neurosci. 2021 Jun 28;15:638439. doi: 10.3389/fncel.2021.638439. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34276307 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

