Shifting Developmental Trajectories During Critical Periods of Brain Formation
- PMID: 33132842
- PMCID: PMC7513795
- DOI: 10.3389/fncel.2020.00283
Shifting Developmental Trajectories During Critical Periods of Brain Formation
Abstract
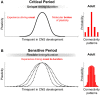
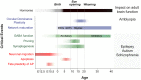
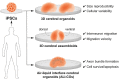
Critical periods of brain development are epochs of heightened plasticity driven by environmental influence necessary for normal brain function. Recent studies are beginning to shed light on the possibility that timely interventions during critical periods hold potential to reorient abnormal developmental trajectories in animal models of neurological and neuropsychiatric disorders. In this review, we re-examine the criteria defining critical periods, highlighting the recently discovered mechanisms of developmental plasticity in health and disease. In addition, we touch upon technological improvements for modeling critical periods in human-derived neural networks in vitro. These scientific advances associated with the use of developmental manipulations in the immature brain of animal models are the basic preclinical systems that will allow the future translatability of timely interventions into clinical applications for neurodevelopmental disorders such as intellectual disability, autism spectrum disorders (ASD) and schizophrenia.
Keywords: brain organoids; critical period; development; neurodevelopmental disorders; plasticity.
Copyright © 2020 Dehorter and Del Pino.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

