Insulin-Like Growth Factor 1 on the Maintenance of Ribbon Synapses in Mouse Cochlear Explant Cultures
- PMID: 33132846
- PMCID: PMC7579230
- DOI: 10.3389/fncel.2020.571155
Insulin-Like Growth Factor 1 on the Maintenance of Ribbon Synapses in Mouse Cochlear Explant Cultures
Abstract
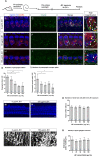
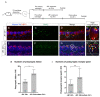
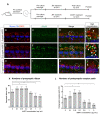
Hearing loss has become one of the most common disabilities worldwide. The synaptic connections between inner hair cells (IHCs) and spiral ganglion neurons have specialized synaptic constructions, termed ribbon synapses, which are important for auditory function. The ribbon synapses in the cochlea are quite vulnerable to various insults. As such, the maintenance of ribbon synapses is important for ensuring hearing function. Insulin-like growth factor 1 (IGF1) plays a critical role in the development and maintenance of the cochlea and has the potential to protect cochlear hair cells from various insults. In this study, we examined the role of IGF1 in the maintenance of ribbon synapses in cochlear explants of postnatal day four mice. We cultured cochlear explants with an IGF1 receptor antagonist, JB1, which is an IGF1 peptide analog. Results showed that exposure to JB1 for 24 h resulted in the loss of ribbon synapses. After an additional 24-h culture without JB1, the number of ribbon synapses spontaneously recovered. The application of exogenous IGF1 showed two different aspects of ribbon synapses. Low doses of exogenous IGF1 promoted the recovery of ribbon synapses, while it compromised the spontaneous recovery of ribbon synapses at high doses. Altogether, these results indicate that the paracrine or autocrine release of IGF1 in the cochlea plays a crucial role in the maintenance of cochlear ribbon synapses.
Keywords: cochlea; inner hair cell; insulin-like growth factor 1; maintenance; ribbon synapse.
Copyright © 2020 Gao, Kita, Katsuno, Yamamoto, Omori and Nakagawa.
Figures
References
-
- Allahdadi K. J., de Santana T. A., Santos G. C., Azevedo C. M., Mota R. A., Nonaka C. K., et al. . (2019). IGF-1 overexpression improves mesenchymal stem cell survival and promotes neurological recovery after spinal cord injury. Stem Cell Res. Ther. 10:146. 10.1186/s13287-019-1223-z - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

