Pain in Endometriosis
- PMID: 33132854
- PMCID: PMC7573391
- DOI: 10.3389/fncel.2020.590823
Pain in Endometriosis
Abstract
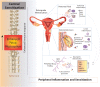
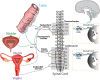
Endometriosis is a chronic and debilitating condition affecting ∼10% of women. Endometriosis is characterized by infertility and chronic pelvic pain, yet treatment options remain limited. In many respects this is related to an underlying lack of knowledge of the etiology and mechanisms contributing to endometriosis-induced pain. Whilst many studies focus on retrograde menstruation, and the formation and development of lesions in the pathogenesis of endometriosis, the mechanisms underlying the associated pain remain poorly described. Here we review the recent clinical and experimental evidence of the mechanisms contributing to chronic pain in endometriosis. This includes the roles of inflammation, neurogenic inflammation, neuroangiogenesis, peripheral sensitization and central sensitization. As endometriosis patients are also known to have co-morbidities such as irritable bowel syndrome and overactive bladder syndrome, we highlight how common nerve pathways innervating the colon, bladder and female reproductive tract can contribute to co-morbidity via cross-organ sensitization.
Keywords: chronic pelvic pain; hyperalgesia; inflammation; neuroangiogenesis; peripheral sensitization; sensory afferents; uterus; vagina.
Copyright © 2020 Maddern, Grundy, Castro and Brierley.
Figures
References
-
- Adamson G., Kennedy S., Hummelshoj L. (2010). Creating solutions in endometriosis: global collaboration through the World Endometriosis Research Foundation. J. Endometr. 2 3–6. 10.1177/228402651000200102 - DOI
-
- Allen C., Hopewell S., Prentice A. (2005). Non-steroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst. Rev. 1:CD004753. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

