Insidious Transmission of a Stress-Related Neuroadaptation
- PMID: 33132859
- PMCID: PMC7571264
- DOI: 10.3389/fnbeh.2020.564054
Insidious Transmission of a Stress-Related Neuroadaptation
Abstract
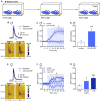
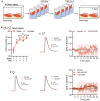
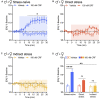
Stress is highly pervasive in humans, impacting motivated behaviors with an enormous toll on life quality. Many of the effects of stress are orchestrated by neuropeptides such as corticotropin-releasing factor (CRF). It has previously been shown that in stress-naïve male mice, CRF acts in the core of the nucleus accumbens (NAc) to produce appetitive effects and to increase dopamine release; yet in stress-exposed male mice, CRF loses its capacity to modulate NAc dopamine release and is aversive. In the current research, we tested whether this effect is comparable in females to males and whether the neuroadaptation is susceptible to social transmission. We found that, like in males, CRF increased dopamine release in stress-naïve but not stress-exposed female mice. Importantly, this persistent physiological change was not accompanied by overt behavioral changes that would be indicative of depression- or anxiety-like phenotype. Nonetheless, when these mice were housed for 7 days with stress-naïve conspecifics, the cage mates also exhibited a loss of dopamine potentiation by CRF. These data demonstrate the asymptomatic, yet pervasive transmission of stress-related neuroadaptations in the population.
Keywords: CRF; dopamine; fast-scan cyclic voltammetry; nucleus accumbens; social stress; stress; stress transmission.
Copyright © 2020 Steger, Land, Lemos, Chavkin and Phillips.
Figures


References
-
- American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th Edn. Arlington, PA: American Psychiatric Association.
-
- Bangasser D. A., Curtis A., Reyes B. A. S., Bethea T. T., Parastatidis I., Ischiropoulos H., et al. (2010). Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry 15, 896–904. 10.1038/mp.2010.66 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

