Ofloxacin as a Disruptor of Actin Aggresome "Hirano Bodies": A Potential Repurposed Drug for the Treatment of Neurodegenerative Diseases
- PMID: 33132905
- PMCID: PMC7573105
- DOI: 10.3389/fnagi.2020.591579
Ofloxacin as a Disruptor of Actin Aggresome "Hirano Bodies": A Potential Repurposed Drug for the Treatment of Neurodegenerative Diseases
Abstract
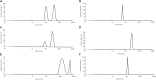
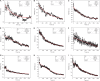
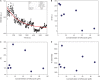
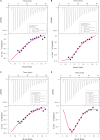
There is a growing number of aging populations that are more prone to the prevalence of neuropathological disorders. Two major diseases that show a late onset of the symptoms include Alzheimer's disorder (AD) and Parkinson's disorder (PD), which are causing an unexpected social and economic impact on the families. A large number of researches in the last decade have focused upon the role of amyloid precursor protein, Aβ-plaque, and intraneuronal neurofibrillary tangles (tau-proteins). However, there is very few understanding of actin-associated paracrystalline structures formed in the hippocampus region of the brain and are called Hirano bodies. These actin-rich inclusion bodies are known to modulate the synaptic plasticity and employ conspicuous effects on long-term potentiation and paired-pulse paradigms. Since the currently known drugs have very little effect in controlling the progression of these diseases, there is a need to develop therapeutic agents, which can have improved efficacy and bioavailability, and can transport across the blood-brain barrier. Moreover, finding novel targets involving compound screening is both laborious and is an expensive process in itself followed by equally tedious Food and Drug Administration (FDA) approval exercise. Finding alternative functions to the already existing FDA-approved molecules for reversing the progression of age-related proteinopathies is of utmost importance. In the current study, we decipher the role of a broad-spectrum general antibiotic (Ofloxacin) on actin polymerization dynamics using various biophysical techniques like right-angle light scattering, dynamic light scattering, circular dichroism spectrometry, isothermal titration calorimetry, scanning electron microscopy, etc. We have also performed in silico docking studies to deduce a plausible mechanism of the drug binding to the actin. We report that actin gets disrupted upon binding to Ofloxacin in a concentration-dependent manner. We have inferred that Ofloxacin, when attached to a drug delivery system, can act as a good candidate for the treatment of neuropathological diseases.
Keywords: SEM; actin; biophysical studies; in silico; neurodegenerative diseases; ofloxacin; repurposable drugs.
Copyright © 2020 Pathak, Parkar, Tripathi and Kale.
Figures







Similar articles
-
Effect of tetracycline family of antibiotics on actin aggregation, resulting in the formation of Hirano bodies responsible for neuropathological disorders.J Biomol Struct Dyn. 2021 Jan;39(1):236-253. doi: 10.1080/07391102.2020.1717629. Epub 2020 Jan 28. J Biomol Struct Dyn. 2021. PMID: 31948361
-
Hirano body expression impairs spatial working memory in a novel mouse model.Acta Neuropathol Commun. 2014 Sep 2;2:131. doi: 10.1186/s40478-014-0131-9. Acta Neuropathol Commun. 2014. PMID: 25178488 Free PMC article.
-
Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: A review.Eur J Med Chem. 2019 May 1;169:185-199. doi: 10.1016/j.ejmech.2019.03.009. Epub 2019 Mar 8. Eur J Med Chem. 2019. PMID: 30877973 Review.
-
Hirano bodies differentially modulate cell death induced by tau and the amyloid precursor protein intracellular domain.BMC Neurosci. 2014 Jun 14;15:74. doi: 10.1186/1471-2202-15-74. BMC Neurosci. 2014. PMID: 24929931 Free PMC article.
-
Neuropathology of Alzheimer's Disease.Neurotherapeutics. 2022 Jan;19(1):173-185. doi: 10.1007/s13311-021-01146-y. Epub 2021 Nov 2. Neurotherapeutics. 2022. PMID: 34729690 Free PMC article. Review.
References
-
- Abraham M. J., Murtola T., Schulz R., Páll S., Smith J. C., Hess B., et al. (2015). Gromacs: high-performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2 19–25. 10.1016/j.softx.2015.06.001 - DOI
LinkOut - more resources
Full Text Sources

