Antipsychotic Withdrawal Symptoms: A Systematic Review and Meta-Analysis
- PMID: 33132934
- PMCID: PMC7552943
- DOI: 10.3389/fpsyt.2020.569912
Antipsychotic Withdrawal Symptoms: A Systematic Review and Meta-Analysis
Abstract
Objective: Avoiding withdrawal symptoms following antipsychotic discontinuation is an important factor when planning a safe therapy. We performed a systematic review and meta-analysis concerning occurrence of withdrawal symptoms after discontinuation of antipsychotics.
Data sources: We searched the databases CENTRAL, Pubmed, and EMBASE with no restriction to the beginning of the searched time period and until October 1, 2019 (PROSPERO registration no. CRD42019119148).
Study selection: Of the 18,043 screened studies, controlled and cohort trials that assessed withdrawal symptoms after discontinuation of oral antipsychotics were included in the random-effects model. Studies that did not implement placebo substitution were excluded from analyses. The primary outcome was the proportion of individuals with withdrawal symptoms after antipsychotic discontinuation. We compared a control group with continued antipsychotic treatment in the assessment of odds ratio and number needed to harm (NNH).
Data extraction: We followed guidelines by the Cochrane Collaboration, PRISMA, and MOOSE.
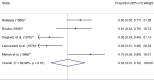
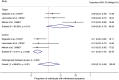
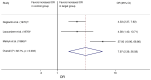
Results: Five studies with a total of 261 individuals were included. The primary outcome, proportion of individuals with withdrawal symptoms after antipsychotic discontinuation, was 0.53 (95% CI, 0.37-0.70; I2 = 82.98%, P < 0.01). An odds ratio of 7.97 (95% CI, 2.39-26.58; I2 = 82.7%, P = 0.003) and NNH of 3 was calculated for the occurrence of withdrawal symptoms after antipsychotic discontinuation.
Conclusion: Withdrawal symptoms appear to occur frequently after abrupt discontinuation of an oral antipsychotic. The lack of randomized controlled trials with low risk of bias on antipsychotic withdrawal symptoms highlights the need for further research.
Keywords: antipsychotics; discontinuation symptoms; meta-analysis; systematic review; withdrawal symptoms.
Copyright © 2020 Brandt, Bschor, Henssler, Müller, Hasan, Heinz and Gutwinski.
Figures
Similar articles
-
Adverse events after antipsychotic discontinuation: an individual participant data meta-analysis.Lancet Psychiatry. 2022 Mar;9(3):232-242. doi: 10.1016/S2215-0366(22)00014-1. Lancet Psychiatry. 2022. PMID: 35183280
-
Antipsychotics for primary alcohol dependence: a systematic review and meta-analysis of placebo-controlled trials.J Clin Psychiatry. 2013 Jul;74(7):e642-54. doi: 10.4088/JCP.12r08178. J Clin Psychiatry. 2013. PMID: 23945459
-
Antipsychotics for cocaine or psychostimulant dependence: systematic review and meta-analysis of randomized, placebo-controlled trials.J Clin Psychiatry. 2013 Dec;74(12):e1169-80. doi: 10.4088/JCP.13r08525. J Clin Psychiatry. 2013. PMID: 24434105
-
Association With Hospitalization and All-Cause Discontinuation Among Patients With Schizophrenia on Clozapine vs Other Oral Second-Generation Antipsychotics: A Systematic Review and Meta-analysis of Cohort Studies.JAMA Psychiatry. 2019 Oct 1;76(10):1052-1062. doi: 10.1001/jamapsychiatry.2019.1702. JAMA Psychiatry. 2019. PMID: 31365048 Free PMC article.
-
Discontinuation of Long-Term Antipsychotic Drug Use for Behavioral and Psychological Symptoms in Older Adults Aged 65 Years and Older With Dementia.J Am Med Dir Assoc. 2018 Nov;19(11):1009-1014. doi: 10.1016/j.jamda.2018.06.023. Epub 2018 Aug 9. J Am Med Dir Assoc. 2018. PMID: 30100234
Cited by
-
Discontinuation of psychotropic medication: a synthesis of evidence across medication classes.Mol Psychiatry. 2024 Aug;29(8):2575-2586. doi: 10.1038/s41380-024-02445-4. Epub 2024 Mar 19. Mol Psychiatry. 2024. PMID: 38503923 Free PMC article. Review.
-
Delirium in Neurocritical Care: Uncovering Undisclosed Psychotropic Substance and Medication Use and Stress Exposure by Hair Analysis.Neurocrit Care. 2025 Feb;42(1):164-174. doi: 10.1007/s12028-024-02052-9. Epub 2024 Jul 16. Neurocrit Care. 2025. PMID: 39009940 Free PMC article.
-
Oral self-administration of pregabalin in a mouse model and the resulting drug addiction features.Saudi Pharm J. 2024 Feb;32(2):101935. doi: 10.1016/j.jsps.2023.101935. Epub 2023 Dec 23. Saudi Pharm J. 2024. PMID: 38292403 Free PMC article.
-
Alliance for Sleep Clinical Practice Guideline on Switching or Deprescribing Hypnotic Medications for Insomnia.J Clin Med. 2023 Mar 25;12(7):2493. doi: 10.3390/jcm12072493. J Clin Med. 2023. PMID: 37048577 Free PMC article. Review.
-
From theory to practice: challenges and rewards of implementing ketogenic metabolic therapy in mental health.Front Nutr. 2024 Feb 8;11:1331181. doi: 10.3389/fnut.2024.1331181. eCollection 2024. Front Nutr. 2024. PMID: 38389794 Free PMC article.
References
-
- Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, Part 2: Update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry (2013) 14:2–44. 10.3109/15622975.2012.739708 - DOI - PubMed
-
- Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet (2019) 394:939–51. 10.1016/S0140-6736(19)31135-3 - DOI - PMC - PubMed
-
- Cerovecki A, Musil R, Klimke A, Seemüller F, Haen E, Schennach R, et al. Withdrawal Symptoms and Rebound Syndromes Associated with Switching and Discontinuing Atypical Antipsychotics: Theoretical Background and Practical Recommendations. CNS Drugs (2013) 27:545–72. 10.1007/s40263-013-0079-5 - DOI - PubMed

