Altered Serum Amino Acid and Acylcarnitine Profiles in Hyperinsulinemic Hypoglycemia and Ketotic Hypoglycemia
- PMID: 33133020
- PMCID: PMC7579424
- DOI: 10.3389/fendo.2020.577373
Altered Serum Amino Acid and Acylcarnitine Profiles in Hyperinsulinemic Hypoglycemia and Ketotic Hypoglycemia
Abstract
Background: In addition to inborn metabolic disorders, altered metabolic profiles were reported to be associated with the risk and prognosis of some non-metabolic diseases, while as a rare metabolic disease, the overall secondary metabolic spectrum in congenital hyperinsulinemic hypoglycemia (HH) is largely undetermined. Therefore, we investigated metabolic profiles in HH patients and used ketotic hypoglycemia (KH) patients as a control cohort to unveil their distinct metabolic features.
Methods: A total of 97 hypoglycemia children, including 74 with hyperinsulinemic hypoglycemia and 23 with ketotic hypoglycemia, and 170 euglycemia control subjects were studied retrospectively. Clinical and biochemical data were collected. The normoglycemic spectra of amino acids and acylcarnitines were determined by liquid chromatography tandem mass spectrometry. The serum insulin and fatty acid concentrations during standardized fasting tests in hypoglycemia patients were also collected. Receiver operating characteristic curve analysis was performed to screen potential biomarkers.
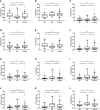
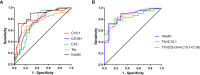
Results: Among the normoglycemic spectra of amino acids, blood valine (p < 0.001), arginine (p < 0.001), threonine (p = 0.001), glutamate (p = 0.002), methionine (p = 0.005), ornithine (p = 0.008), leucine (p = 0.014), alanine (p = 0.017), proline (p = 0.031), citrulline (p = 0.042), aspartate (p = 0.046), and glycine (p = 0.048) levels differed significantly among the three groups. Significantly decreased levels of long- (C14:1, p < 0.001; C18, p < 0.001), medium- (C8, p < 0.001; C10, p < 0.001; C10:1, p < 0.001), and short-chain (C4-OH, p < 0.001; C5OH, p < 0.001) acylcarnitines were found in the hyperinsulinemic hypoglycemia group. Hyperinsulinemic hypoglycemia children with focal lesions and diffuse lesions had similar amino acid and acylcarnitine spectra. C10:1 < 0.09 μmol/L, threonine > 35 μmol/L, and threonine/C10:1 > 440 showed sensitivities of 81.1, 66.2, and 81.1% and specificities of 72.7, 78.3, and 81.8%, respectively, in distinguishing HH from KH.
Conclusions: We found significantly different altered serum amino acid and acylcarnitine profiles at normoglycemia, especially decreased C10:1 and increased threonine levels, between HH and KH children, which may reflect the insulin ketogenesis inhibition effect in HH patients; however, the detailed mechanisms and physiological roles remain to be studied in the future.
Keywords: acylcarnitine; amino acid; hyperinsulinemic hypoglycemia; hypoglycemia; ketotic hypoglycemia.
Copyright © 2020 Xu, Zhu, Lu, Sun, Cheng, Ni, Xi, Hussain, Luo and Zhang.
Figures
Similar articles
-
Biomarkers of Insulin for the Diagnosis of Hyperinsulinemic Hypoglycemia in Infants and Children.J Pediatr. 2016 Jan;168:212-219. doi: 10.1016/j.jpeds.2015.09.045. Epub 2015 Oct 17. J Pediatr. 2016. PMID: 26490124
-
Insulin-like growth factor binding protein-1 levels in the diagnosis of hypoglycemia caused by hyperinsulinism.J Pediatr. 1997 Aug;131(2):193-9. doi: 10.1016/s0022-3476(97)70153-7. J Pediatr. 1997. PMID: 9290603
-
Study of Carnitine/Acylcarnitine and Amino Acid Profile in Children and Adults With Acute Liver Failure.J Pediatr Gastroenterol Nutr. 2017 Jun;64(6):869-875. doi: 10.1097/MPG.0000000000001510. J Pediatr Gastroenterol Nutr. 2017. PMID: 28045774
-
Idiopathic Pathological Ketotic Hypoglycemia: Finding the Needle in a Haystack.Horm Res Paediatr. 2025;98(3):246-257. doi: 10.1159/000538483. Epub 2024 Mar 21. Horm Res Paediatr. 2025. PMID: 38513624 Free PMC article. Review.
-
The Diagnosis and Management of Hyperinsulinaemic Hypoglycaemia.J Clin Res Pediatr Endocrinol. 2015 Jun;7(2):86-97. doi: 10.4274/jcrpe.1891. J Clin Res Pediatr Endocrinol. 2015. PMID: 26316429 Free PMC article. Review.
Cited by
-
Congenital hyperinsulinism in clinical practice: From biochemical pathophysiology to new monitoring techniques.Front Pediatr. 2022 Sep 23;10:901338. doi: 10.3389/fped.2022.901338. eCollection 2022. Front Pediatr. 2022. PMID: 36210928 Free PMC article. Review.
-
A nomogram based on metabolic profiling to discriminate lung cancer among patients with lung nodules.J Int Med Res. 2023 Mar;51(3):3000605231161204. doi: 10.1177/03000605231161204. J Int Med Res. 2023. PMID: 36974888 Free PMC article. Clinical Trial.
References
-
- Thornton PS, Stanley CA, De Leon DD, Harris D, Haymond MW, Hussain K, et al. Pediatric Endocrine Society. Recommendations from the Pediatric Endocrine Society for Evaluation and Management of Persistent Hypoglycemia in Neonates, Infants, and Children. J Pediatr (2015) 167(2):238–45. 10.1016/j.jpeds.2015.03.057 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

