Amelioration of Androgenetic Alopecia by Algal Oligosaccharides Prepared by Deep-Sea Bacterium Biodegradation
- PMID: 33133041
- PMCID: PMC7550528
- DOI: 10.3389/fmicb.2020.567060
Amelioration of Androgenetic Alopecia by Algal Oligosaccharides Prepared by Deep-Sea Bacterium Biodegradation
Abstract
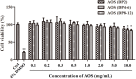
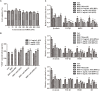
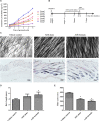
Androgenetic alopecia (AGA) is a dihydrotestosterone (DHT)-mediated hair loss disorder characterized by shortened anagen hair cycle. Oligosaccharides derived from seaweeds possess diverse biological functions. However, little is known about their effects on AGA. In this study, algal oligosaccharide (AOS) was characterized for its mitigation effects on key features involved in AGA pathogenesis, such as DHT- mediated cellular signaling and shortened anagen hair cycle. AOS with varying degrees of polymerization (DP), namely, AOS (DP2), AOS (DP4-6), and AOS (DP8-12), were prepared by agar biodegradation with Flammeovirga pacifica WPAGA1, an agarolytic bacterium isolated from deep-sea sediments. In vitro results showed that AOS with varying DPs significantly ameliorated the DHT-induced alterations of regulatory factors in human hair follicle dermal papilla cells in a dose- and DP-dependent manner, as revealed by the normalization of several hair-growth-stimulating or inhibitory factors. In vivo studies showed that AOS (DP2) extended the anagen phase and thereby delayed catagen progression in mice. Furthermore, AOS (DP2) stimulated dorsal hair growth in mice by increasing hair length, density, and thickness. Therefore, our findings indicated that AOS antagonized key factors involved in AGA pathogenesis, suggesting the potential application of AOS in the prevention and the treatment of AGA.
Keywords: algal oligosaccharides; anagen; androgenetic alopecia; deep-sea bacterium; dihydrotestosterone.
Copyright © 2020 Jin, Chen, He, Hou, Chan and Zeng.
Figures

Similar articles
-
Efficacy of Asymmetric siRNA Targeting Androgen Receptors for the Treatment of Androgenetic Alopecia.Mol Pharm. 2023 Jan 2;20(1):128-135. doi: 10.1021/acs.molpharmaceut.2c00510. Epub 2022 Nov 9. Mol Pharm. 2023. PMID: 36352823 Free PMC article.
-
Increased Expression of Zyxin and Its Potential Function in Androgenetic Alopecia.Front Cell Dev Biol. 2021 Jan 11;8:582282. doi: 10.3389/fcell.2020.582282. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33505959 Free PMC article.
-
Cell-free fat extract restores hair loss: a novel therapeutic strategy for androgenetic alopecia.Stem Cell Res Ther. 2023 Aug 23;14(1):219. doi: 10.1186/s13287-023-03398-1. Stem Cell Res Ther. 2023. PMID: 37612726 Free PMC article.
-
Androgen modulation of Wnt/β-catenin signaling in androgenetic alopecia.Arch Dermatol Res. 2018 Jul;310(5):391-399. doi: 10.1007/s00403-018-1826-8. Epub 2018 Mar 16. Arch Dermatol Res. 2018. PMID: 29549490 Review.
-
Molecular basis of androgenetic alopecia: From androgen to paracrine mediators through dermal papilla.J Dermatol Sci. 2011 Jan;61(1):1-6. doi: 10.1016/j.jdermsci.2010.10.015. Epub 2010 Nov 3. J Dermatol Sci. 2011. PMID: 21167691 Review.
Cited by
-
A combination therapy for androgenic alopecia based on quercetin and zinc/copper dual-doped mesoporous silica nanocomposite microneedle patch.Bioact Mater. 2022 Dec 13;24:81-95. doi: 10.1016/j.bioactmat.2022.12.007. eCollection 2023 Jun. Bioact Mater. 2022. PMID: 36582348 Free PMC article.
-
Application of Marine Microbial Natural Products in Cosmetics.Front Microbiol. 2022 May 26;13:892505. doi: 10.3389/fmicb.2022.892505. eCollection 2022. Front Microbiol. 2022. PMID: 35711762 Free PMC article. Review.
-
Mannan oligosaccharides improve the fur quality of raccoon dogs by regulating the gut microbiota.Front Microbiol. 2023 Dec 15;14:1324277. doi: 10.3389/fmicb.2023.1324277. eCollection 2023. Front Microbiol. 2023. PMID: 38169639 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

