Antimicrobial Peptide Induced-Stress Renders Staphylococcus aureus Susceptible to Toxic Nucleoside Analogs
- PMID: 33133056
- PMCID: PMC7550632
- DOI: 10.3389/fimmu.2020.01686
Antimicrobial Peptide Induced-Stress Renders Staphylococcus aureus Susceptible to Toxic Nucleoside Analogs
Abstract
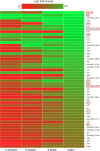
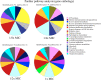
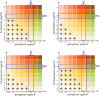
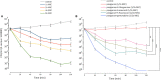
Cationic antimicrobial peptides (AMPs) are active immune effectors of multicellular organisms and are also considered as new antimicrobial drug candidates. One of the problems encountered when developing AMPs as drugs is the difficulty of reaching sufficient killing concentrations under physiological conditions. Here, using pexiganan, a cationic peptide derived from a host defense peptide of the African clawed frog and the first AMP developed into an antibacterial drug, we studied whether sub-lethal effects of AMPs can be harnessed to devise treatment combinations. We studied the pexiganan stress response of Staphylococcus aureus at sub-lethal concentrations using quantitative proteomics. Several proteins involved in nucleotide metabolism were elevated, suggesting a metabolic demand. We then show that Staphylococcus aureus is highly susceptible to antimetabolite nucleoside analogs when exposed to pexiganan, even at sub-inhibitory concentrations. These findings could be used to enhance pexiganan potency while decreasing the risk of resistance emergence, and our findings can likely be extended to other antimicrobial peptides.
Keywords: antibiotic resistance; antibiotics; antimetabolites; antimicrobial peptides; nuceloside analogs; pexiganan.
Copyright © 2020 Rodríguez-Rojas, Nath, El Shazely, Santi, Kim, Weise, Kuropka and Rolff.
Figures
Similar articles
-
Genomic Signatures of Experimental Adaptation to Antimicrobial Peptides in Staphylococcus aureus.G3 (Bethesda). 2016 Jun 1;6(6):1535-9. doi: 10.1534/g3.115.023622. G3 (Bethesda). 2016. PMID: 27172179 Free PMC article.
-
Role of the LytSR two-component regulatory system in adaptation to cationic antimicrobial peptides in Staphylococcus aureus.Antimicrob Agents Chemother. 2013 Aug;57(8):3875-82. doi: 10.1128/AAC.00412-13. Epub 2013 Jun 3. Antimicrob Agents Chemother. 2013. PMID: 23733465 Free PMC article.
-
Comparing selection on S. aureus between antimicrobial peptides and common antibiotics.PLoS One. 2013 Oct 18;8(10):e76521. doi: 10.1371/journal.pone.0076521. eCollection 2013. PLoS One. 2013. PMID: 24204634 Free PMC article.
-
Pexiganan acetate.Drugs. 1998 Dec;56(6):1047-52; discussion 1053-4. doi: 10.2165/00003495-199856060-00011. Drugs. 1998. PMID: 9878992 Review.
-
Antimicrobial Peptides: the Achilles' Heel of Antibiotic Resistance?Probiotics Antimicrob Proteins. 2019 Jun;11(2):370-381. doi: 10.1007/s12602-018-9465-0. Probiotics Antimicrob Proteins. 2019. PMID: 30229514 Review.
Cited by
-
Metabolomic Profiling and Anti-Helicobacter pylori Activity of Caulerpa lentillifera (Sea Grape) Extract.Mar Drugs. 2025 Jul 7;23(7):282. doi: 10.3390/md23070282. Mar Drugs. 2025. PMID: 40710507 Free PMC article.
-
The evolution of antimicrobial peptide resistance in Pseudomonas aeruginosa is severely constrained by random peptide mixtures.PLoS Biol. 2024 Jul 2;22(7):e3002692. doi: 10.1371/journal.pbio.3002692. eCollection 2024 Jul. PLoS Biol. 2024. PMID: 38954678 Free PMC article.
-
Halogenated Pyrrolopyrimidines with Low MIC on Staphylococcus aureus and Synergistic Effects with an Antimicrobial Peptide.Antibiotics (Basel). 2022 Jul 22;11(8):984. doi: 10.3390/antibiotics11080984. Antibiotics (Basel). 2022. PMID: 35892374 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

