Comparative Analysis of G1P[8] Rotaviruses Identified Prior to Vaccine Implementation in Pakistan With Rotarix™ and RotaTeq™ Vaccine Strains
- PMID: 33133073
- PMCID: PMC7562811
- DOI: 10.3389/fimmu.2020.562282
Comparative Analysis of G1P[8] Rotaviruses Identified Prior to Vaccine Implementation in Pakistan With Rotarix™ and RotaTeq™ Vaccine Strains
Erratum in
-
Corrigendum: Comparative Analysis of G1P[8] Rotaviruses Identified Prior to Vaccine Implementation in Pakistan With Rotarix™ and RotaTeq™ Vaccine Strains.Front Immunol. 2020 Dec 16;11:625157. doi: 10.3389/fimmu.2020.625157. eCollection 2020. Front Immunol. 2020. PMID: 33391290 Free PMC article.
Abstract
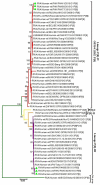
Group A rotavirus (RVA) is the leading cause of severe childhood diarrhea globally, even with all effective interventions, particularly in developing countries. Among the diverse genotypes of RVA, G1P[8] is a common genotype that has continued to pervade around the world, including Pakistan. Two universally accepted rotavirus vaccines-Rotarix™ and RotaTeq™ contain the genotype G1P[8]. The current work was aimed at identifying differences between antigenic epitopes of Pakistan's G1P[8] strains and those of the two licensed vaccines. We sequenced 6 G1P[8] rotavirus strains previously reported in Rawalpindi, Islamabad, Pakistan in 2015 and 2016 for their outer capsid genes (VP7 and VP4). Phylogenetic analysis was then conducted in order to classify their specific lineages and to detect their association with strains isolated throughout world. Compared with the Rotarix™ and RotaTeq™ vaccine strains (G1-lineage II, P[8]-lineage III), our study G1-lineage I, P[8]-lineage IV strains showed 3 and 5 variations in the VP7 epitopes, respectively, and 13 and 11 variations in the VP4 epitopes, respectively. The G1 lineage II strains showed no single amino acid change compared to Rotarix™ (lineage II), but exhibited changes at 2 positions compared to RotaTeq™ (lineage III). So, this has been proposed that these G1 strains exist in our natural setting, or that they may have been introduced in Pakistan from other countries of the world. The distinct P[8]-lineage IV (OP354-like) strains showed twelve and thirteen amino acid variations, with Rotarix™ and RotaTeq™ (lineages II and III) strains, respectively. Such findings have shown that the VP4-P[8] component of the G1P[8] strains circulating in Pakistan differs considerably from that of the vaccine viruses compared to that of the VP7-G1. To monitor the long-term effects of vaccines on the emergence of G1P[8] strains with different lineages, routine and successful monitoring of these strains will be crucial.
Keywords: G1P[8]; antigenic epitopes; emergence; genotype; rotavirus.
Copyright © 2020 Sadiq and Bostan.
Figures




Similar articles
-
Sequence analysis of VP7 and VP4 genes of G1P[8] rotaviruses circulating among diarrhoeic children in Pune, India: a comparison with Rotarix and RotaTeq vaccine strains.Vaccine. 2014 Aug 11;32 Suppl 1:A75-83. doi: 10.1016/j.vaccine.2014.03.080. Vaccine. 2014. PMID: 25091685
-
Genetic variability of VP7, VP4, VP6 and NSP4 genes of common human G1P[8] rotavirus strains circulating in Italy between 2010 and 2014.Virus Res. 2016 Jul 15;220:117-28. doi: 10.1016/j.virusres.2016.04.018. Epub 2016 Apr 26. Virus Res. 2016. PMID: 27130628
-
Genetic analyses reveal differences in the VP7 and VP4 antigenic epitopes between human rotaviruses circulating in Belgium and rotaviruses in Rotarix and RotaTeq.J Clin Microbiol. 2012 Mar;50(3):966-76. doi: 10.1128/JCM.05590-11. Epub 2011 Dec 21. J Clin Microbiol. 2012. PMID: 22189107 Free PMC article.
-
Genetic diversity of G1P[8] rotavirus VP7 and VP8* antigens in Finland over a 20-year period: No evidence for selection pressure by universal mass vaccination with RotaTeq® vaccine.Infect Genet Evol. 2013 Oct;19:51-8. doi: 10.1016/j.meegid.2013.06.026. Epub 2013 Jul 4. Infect Genet Evol. 2013. PMID: 23831933 Review.
-
Circulating rotavirus strains in children with acute gastroenteritis in Iran, 1986 to 2023 and their genetic/antigenic divergence compared to approved vaccines strains (Rotarix, RotaTeq, ROTAVAC, ROTASIIL) before mass vaccination: Clues for vaccination policy makers.Virus Res. 2024 Aug;346:199411. doi: 10.1016/j.virusres.2024.199411. Epub 2024 Jun 3. Virus Res. 2024. PMID: 38823689 Free PMC article. Review.
Cited by
-
Phylogenetic analysis of the viral proteins VP4/VP7 of circulating human rotavirus strains in China from 2016 to 2019 and comparison of their antigenic epitopes with those of vaccine strains.Front Cell Infect Microbiol. 2022 Aug 8;12:927490. doi: 10.3389/fcimb.2022.927490. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36004332 Free PMC article.
-
Vaccine evaluation and genotype characterization in children infected with rotavirus in Qatar.Pediatr Res. 2023 Aug;94(2):477-485. doi: 10.1038/s41390-023-02468-7. Epub 2023 Jan 19. Pediatr Res. 2023. PMID: 36658331 Free PMC article.
-
Vaccinomics Approach for Multi-Epitope Vaccine Design against Group A Rotavirus Using VP4 and VP7 Proteins.Vaccines (Basel). 2023 Mar 24;11(4):726. doi: 10.3390/vaccines11040726. Vaccines (Basel). 2023. PMID: 37112638 Free PMC article.
-
Rotavirus prevalence and genotypes in the Central African Republic, 2011-2021.BMC Infect Dis. 2025 May 8;25(1):681. doi: 10.1186/s12879-025-11057-4. BMC Infect Dis. 2025. PMID: 40340659 Free PMC article.
-
Rotavirus gastroenteritis in Pakistan, 2018: updated disease burden.BMC Infect Dis. 2021 May 6;21(1):426. doi: 10.1186/s12879-021-06123-6. BMC Infect Dis. 2021. PMID: 33957883 Free PMC article.
References
-
- Tate JE, Burton AH, Boschi-Pinto C, Parashar UD, World Health Organization–Coordinated Global Rotavirus Surveillance Network for the WHOGRS. Agocs M, et al. Global, Regional, and National Estimates of Rotavirus Mortality in Children <5 Years of Age, 2000–2013. Clin Infect Dis (2016) 62(Suppl 2):S96–S105. 10.1093/cid/civ1013 - DOI - PMC - PubMed
-
- WHO Estimated rotavirus deaths for children under 5 years of age: 2013, 215 000. WHO; (2016). Available at: https://www.who.int/immunization/monitoring_surveillance/burden/estimate... (Accessed October 24, 2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

