A Retrospective Analysis of Full-face Dermal Filler Treatments: Product Choice, Volume Use, and Treatment Locations
- PMID: 33133339
- PMCID: PMC7577331
A Retrospective Analysis of Full-face Dermal Filler Treatments: Product Choice, Volume Use, and Treatment Locations
Abstract
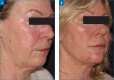
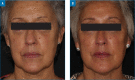
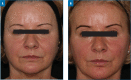
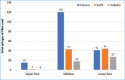
OBJECTIVE: The Vycross™ range of hyaluronic acid (HA) fillers supports a full-face approach to facial rejuvenation. It is important to understand how much filler can be safely injected and which products to use where, particularly for injectors who are new to this range. This study assessed whether Vycross fillers can be effectively and safely used in larger quantities and tracked real-world usage across facial zones. METHODS: This was a single-center, retrospective analysis set in normal clinical practice. The study included 66 consecutive adult female patients undergoing full-face rejuvenation with Vycross fillers within a single treatment plan guided by MD Codes. Filler usage and location were noted, and any adverse events were recorded. RESULTS: The mean age was 48.0 years, and median time from consultation to first filler usage was 18 days. Most patients (n=40; 60.6%) had only one filler session. Forty-seven patients (71.2%) also received botulinum toxin type A. In total, 309 filler syringes were used (mean: 4.7 per patient). Sixteen (5.2%), 181 (58.6%), and 112 (36.2%) syringes were used in the upper, mid and lower face, respectively. In the upper and midface, the majority was Voluma (15/16 and 120/181 syringes, respectively). In the lower face, filler use was split across Voluma, Volift and Volbella (41, 44 and 27 syringes, respectively). Five adverse events were reported at three weeks (swelling, n=2; asymmetry, n=2; lumps, n=1). All were easily resolved. No delayed events were reported. CONCLUSION: Substantial volumes of Vycross fillers can be effectively and safely used as part of a full-face approach to facial rejuvenation.
Keywords: Vycross; dermal filler; facial rejuvenation; hyaluronic acid.
Copyright © 2020. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
FUNDING:This study was funded by Allergan. DISCLOSURES:Dr. Dhillon is a former employee of Allergan, an Allergan stockholder, and a consultant for Allergan. Dr. Patel is a consultant for Allergan.
Figures


References
-
- Rohrich RJ, Pessa JE. The fat compartments of the face: Anatomy and clinical implications for cosmetic surgery. Plast Reconstr Surg. 2007;119:2219–2231. - PubMed
-
- Bartlett SP, Grossman R, Whitaker LA. Age-related changes of the craniofacial skeleton: an anthropomorphic and histologic analysis. Plast Reconstr Surg. 1992;90:592–600. - PubMed
-
- Coleman SR, Grover R. The anatomy of the aging face: volume loss and changes in 3-dimensional topography. Aesthetic Surg J. 2006;26:S4–S9. - PubMed
-
- Forte AJ, Andrew TW, Colasante C, Persing JA. Perception of age, attractiveness, and tiredness after isolated and combined facial subunit aging. Aesth Plast Surg. 2015;39:856–869. - PubMed
-
- Tan SL, Brandt MG, Yeung JC et al. The aesthetic unit principle of facial aging. JAMA Facial Plast Surg. 2015;17:33–38. - PubMed
LinkOut - more resources
Full Text Sources
