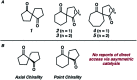
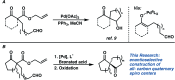
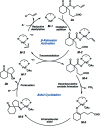
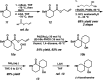
Catalytic enantioselective synthesis of carbocyclic and heterocyclic spiranes via a decarboxylative aldol cyclization
- PMID: 33133488
- PMCID: PMC7574022
- DOI: 10.1039/d0sc02366c
Catalytic enantioselective synthesis of carbocyclic and heterocyclic spiranes via a decarboxylative aldol cyclization
Abstract
The synthesis of a variety of enantioenriched 1,3-diketospiranes from the corresponding racemic allyl β-ketoesters via an interrupted asymmetric allylic alkylation is disclosed. Substrates possessing pendant aldehydes undergo decarboxylative enolate formation in the presence of a chiral Pd catalyst and subsequently participate in an enantio- and diastereoselective, intramolecular aldol reaction to furnish spirocyclic β-hydroxy ketones which may be oxidized to the corresponding enantioenriched diketospiranes. Additionally, this chemistry has been extended to α-allylcarboxy lactam substrates leading to a formal synthesis of the natural product (-)-isonitramine.
This journal is © The Royal Society of Chemistry 2020.
Figures
Similar articles
-
Synthesis of Chiral Nonracemic α-Difluoromethylthio Compounds with Tetrasubstituted Stereogenic Centers via a Palladium-Catalyzed Decarboxylative Asymmetric Allylic Alkylation.Org Lett. 2018 Nov 16;20(22):7044-7048. doi: 10.1021/acs.orglett.8b02998. Epub 2018 Nov 8. Org Lett. 2018. PMID: 30407832
-
Highly Enantioselective Formation of α-Allyl-α-Arylcyclopentanones via Pd-Catalysed Decarboxylative Asymmetric Allylic Alkylation.Chemistry. 2016 Jul 11;22(29):9938-42. doi: 10.1002/chem.201602250. Epub 2016 Jun 14. Chemistry. 2016. PMID: 27191198
-
Asymmetric synthesis of α-trifluoromethoxy ketones with a tetrasubstituted α-stereogenic centre via the palladium-catalyzed decarboxylative allylic alkylation of allyl enol carbonates.Chem Commun (Camb). 2018 May 29;54(44):5522-5525. doi: 10.1039/c8cc03131b. Chem Commun (Camb). 2018. PMID: 29767203
-
[Development of highly stereoselective reactions utilizing heteroatoms--asymmetric synthesis of alpha-substituted serines].Yakugaku Zasshi. 2000 Jan;120(1):28-41. doi: 10.1248/yakushi1947.120.1_28. Yakugaku Zasshi. 2000. PMID: 10655780 Review. Japanese.
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
Cited by
-
Divergent Catalysis: Catalytic Asymmetric [4+2] Cycloaddition of Palladium Enolates.J Am Chem Soc. 2023 May 24;145(20):11301-11310. doi: 10.1021/jacs.3c02104. Epub 2023 May 15. J Am Chem Soc. 2023. PMID: 37186945 Free PMC article.
-
Enantioselective Spirocyclization of Pd-Enolates and Isocyanates.Angew Chem Int Ed Engl. 2025 Jun 2;64(23):e202502583. doi: 10.1002/anie.202502583. Epub 2025 Apr 17. Angew Chem Int Ed Engl. 2025. PMID: 40148236
-
SPA-PNN ligand for the kinetic resolution of carbocyclic and heterocyclic spiranes by asymmetric hydrogenation.Nat Commun. 2025 Jul 2;16(1):6078. doi: 10.1038/s41467-025-61360-6. Nat Commun. 2025. PMID: 40603305 Free PMC article.
-
Enantioselective Nickel-Catalyzed α-Spirocyclization of Lactones.Org Lett. 2024 Aug 16;26(32):6793-6797. doi: 10.1021/acs.orglett.4c01661. Epub 2024 Aug 1. Org Lett. 2024. PMID: 39087908 Free PMC article.
References
-
-
For reviews of enantioselective synthesis of spirocyclic compounds, see:
- Rios R. Chem. Soc. Rev. 2012;41:1060. - PubMed
- Franz A. K., Hanhan N. V., Ball-Jones N. R. ACS Catal. 2013;3:540.
- Ball-Jones N. R., Badillo J. J., Franz A. K. Org. Biomol. Chem. 2012;10:5165. - PubMed
- Trost B. M., Brennan M. Synthesis. 2009;18:3003.
- Cheng D., Ishihara Y., Tan B., Barbas III C. F. ACS Catal. 2014;4:743.
-
-
-
For general reviews of synthetic strategies toward spirocycles, see:
- Smith L. K., Baxendale I. R. Org. Biomol. Chem. 2015;13:9907. - PubMed
- Krapcho A. P. Synthesis. 1974;6:383.
- Sannigrahi M. Tetrahedron. 1999;55:9007.
- Pradhan R., Patra M., Behera A. K., Mishra B. K., Behera R. K. Tetrahedron. 2006;62:779.
- Galliford C. V., Scheidt K. A. Angew. Chem., Int. Ed. 2007;46:8748. - PubMed
- Kotha S., Deb A. C., Lahiri K., Manivannan E. Synthesis. 2009;2:165.
- Zhuo C.-X., Zheng C., You S.-L. Acc. Chem. Res. 2014;47:2558. - PubMed
- D'yakonov V. A., Trapeznikova O. A., de Meijere A., Dzhemilev U. M. Chem. Rev. 2014;114:5775. - PubMed
-
-
-
For reviews of enantioselective synthesis of all-carbon quaternary stereocenters, see:
- Fuji K. Chem. Rev. 1993;93:2037.
- Corey E. J., Guzman-Perez A. Angew. Chem., Int. Ed. 1998;37:388. - PubMed
- Christoffers J., Mann A. Angew. Chem., Int. Ed. 2001;40:4591. - PubMed
- Denissova I., Barriault L. Tetrahedron. 2003;59:10105.
- Douglas C. J., Overman L. E. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5363. - PMC - PubMed
- Christoffers J., Baro A. Adv. Synth. Catal. 2005;347:1473.
- Trost B. M., Jiang C. Synthesis. 2006;3:369.
- Cozzi P. G., Hilgraf R., Zimmermann N. Eur. J. Org. Chem. 2007;2007:5969.
- Prakash J., Marek I. Chem. Commun. 2011;47:4593. - PubMed
- Quasdorf K. W., Overman L. E. Nature. 2014;516:181. - PMC - PubMed
- Liu Y., Han S.-H., Liu W.-B., Stoltz B. M. Acc. Chem. Res. 2015;48:740. - PMC - PubMed
- Feng J., Holmes M., Krische M. J. Chem. Rev. 2017;117:12564. - PMC - PubMed
- Pierrot D., Marek I. Angew. Chem., Int. Ed. 2019;59:36. - PubMed
-
-
-
For examples of racemic syntheses of 1,3-diketospiranes, see:
- Gerlach H., Muller W. Angew. Chem., Int. Ed. 1972;11:1030.
- Wheeler T. N., Jackson C. A., Young K. H. J. Org. Chem. 1973;39:1318.
- Carruthers W., Orridge A. J. Chem. Soc., Perkin Trans. 1. 1977;21:2411.
-
Grants and funding
LinkOut - more resources
Full Text Sources

