Lysimachia christinae Hance as an anticancer agent against breast cancer cells
- PMID: 33133573
- PMCID: PMC7590289
- DOI: 10.1002/fsn3.1875
Lysimachia christinae Hance as an anticancer agent against breast cancer cells
Abstract
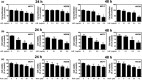
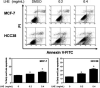
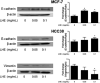
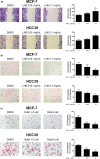
Breast cancer is the most common cancer in women, and metastasis is the leading cause of death in breast cancer patients. Although chemoprevention is widely employed to treat breast cancer, anticancer drugs can cause significant adverse effects. Lysimachia christinae Hance (LH) is a traditional Chinese medicinal plant with diverse therapeutic effects. However, its potential anticancer activity has not been fully investigated in breast cancers to date. Using high-performance liquid chromatography-mass spectrometry, we found that the main constituent of LH extract (LHE) was rutin. Our results indicated that LHE or rutin markedly decreased the proliferation and viability of estrogen receptor (ER)-positive MCF-7 and ER-negative HCC38 human breast cancer cells. LHE treatment induced morphological changes in apoptotic nuclei using 4',6-diamidino-2-phenylindole (DAPI) staining. Annexin V-fluorescein isothiocyanate (FITC) propidium iodide (PI) staining assay revealed that apoptosis significantly increased in both breast cancer cell types after LHE treatment. Additionally, the expression of poly (ADP-ribose) polymerase (PARP), Bcl-2, and phospho-Akt decreased, while that of cleaved PARP and p53 increased, in both cell types. Furthermore, LHE treatment inhibited epithelial-mesenchymal transition (EMT). LHE treatment significantly upregulated E-cadherin level in MCF-7 and HCC38 cells, while vimentin level was downregulated in HCC38 cells. In addition, transwell and wound-healing assays revealed that LHE or rutin inhibited breast cancer cell migration. Overall, these findings demonstrate that LHE is a promising therapeutic agent that acts by promoting apoptosis and reducing cell proliferation, EMT, and cell migration in ER-positive and ER-negative breast cancer cells.
Keywords: Lysimachia christinae Hance; apoptosis; breast cancer; epithelial–mesenchymal transition.
© 2020 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

