Recent progress in the molecular imaging of therapeutic monoclonal antibodies
- PMID: 33133724
- PMCID: PMC7591813
- DOI: 10.1016/j.jpha.2020.07.006
Recent progress in the molecular imaging of therapeutic monoclonal antibodies
Abstract
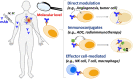
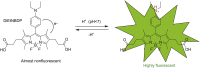
Therapeutic monoclonal antibodies have become one of the central components of the healthcare system and continuous efforts are made to bring innovative antibody therapeutics to patients in need. It is equally critical to acquire sufficient knowledge of their molecular structure and biological functions to ensure the efficacy and safety by incorporating new detection approaches since new challenges like individual differences and resistance are presented. Conventional techniques for determining antibody disposition including plasma drug concentration measurements using LC-MS or ELISA, and tissue distribution using immunohistochemistry and immunofluorescence are now complemented with molecular imaging modalities like positron emission tomography and near-infrared fluorescence imaging to obtain more dynamic information, while methods for characterization of antibody's interaction with the target antigen as well as visualization of its cellular and intercellular behavior are still under development. Recent progress in detecting therapeutic antibodies, in particular, the development of methods suitable for illustrating the molecular dynamics, is described here.
Keywords: Biological function; Molecular imaging; Molecular structure; Therapeutic monoclonal antibodies.
© 2020 Xi'an Jiaotong University. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures





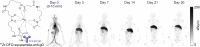



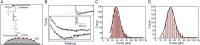





Similar articles
-
Characterization of a recombinant humanized anti-cocaine monoclonal antibody and its Fab fragment.Hum Vaccin Immunother. 2015;11(2):458-67. doi: 10.4161/21645515.2014.990856. Hum Vaccin Immunother. 2015. PMID: 25692880 Free PMC article.
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Attribution of the discrepancy between ELISA and LC-MS/MS assay results of a PEGylated scaffold protein in post-dose monkey plasma samples due to the presence of anti-drug antibodies.Anal Bioanal Chem. 2012 Jan;402(3):1229-39. doi: 10.1007/s00216-011-5527-9. Epub 2011 Dec 1. Anal Bioanal Chem. 2012. PMID: 22130720
-
Molecular Imaging of Pancreatic Cancer with Antibodies.Mol Pharm. 2016 Jan 4;13(1):8-24. doi: 10.1021/acs.molpharmaceut.5b00626. Epub 2015 Dec 10. Mol Pharm. 2016. PMID: 26620581 Free PMC article. Review.
-
Paradigms in Fluorescence Molecular Imaging: Maximizing Measurement of Biological Changes in Disease, Therapeutic Efficacy, and Toxicology/Safety.Mol Imaging Biol. 2019 Aug;21(4):599-611. doi: 10.1007/s11307-018-1273-0. Mol Imaging Biol. 2019. PMID: 30218390 Review.
Cited by
-
124I-labeled anti-CD147 antibody for noninvasive detection of CD147-positive pan-cancers: construction and preclinical studies.Acta Pharmacol Sin. 2024 Feb;45(2):436-448. doi: 10.1038/s41401-023-01162-y. Epub 2023 Sep 25. Acta Pharmacol Sin. 2024. PMID: 37749238 Free PMC article.
-
Real-time imaging of cell-surface proteins with antibody-based fluorogenic probes.Chem Sci. 2021 Sep 16;12(40):13477-13482. doi: 10.1039/d1sc03065e. eCollection 2021 Oct 20. Chem Sci. 2021. PMID: 34777767 Free PMC article.
-
Peptide-based immuno-PET/CT monitoring of dynamic PD-L1 expression during glioblastoma radiotherapy.J Pharm Anal. 2025 Mar;15(3):101082. doi: 10.1016/j.jpha.2024.101082. Epub 2024 Aug 26. J Pharm Anal. 2025. PMID: 40177067 Free PMC article.
-
The Perspective of Therapeutic Antibody Marketing in Iran: Trend and Estimation by 2025.Adv Pharmacol Pharm Sci. 2021 Mar 30;2021:5569590. doi: 10.1155/2021/5569590. eCollection 2021. Adv Pharmacol Pharm Sci. 2021. PMID: 33860229 Free PMC article.
-
Rapid discovery and evolution of nanosensors containing fluorogenic amino acids.Nat Commun. 2024 Sep 5;15(1):7531. doi: 10.1038/s41467-024-50956-z. Nat Commun. 2024. PMID: 39237489 Free PMC article.
References
-
- Carter P.J., Lazar G.A. Next generation antibody drugs: pursuit of the ‘high-hanging fruit’. Nat. Rev. Drug Discov. 2018;17:197–223. - PubMed
-
- Tsumoto K., Isozaki Y., Yagami H. Future perspectives of therapeutic monoclonal antibodies. Immunotherapy. 2019;11:119–127. - PubMed
-
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. - PubMed
-
- Rodgers K.R., Chou R.C. Therapeutic monoclonal antibodies and derivatives: historical perspectives and future directions. Biotechnol. Adv. 2016;34:1149–1158. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

