Biochemical, hematological and histopathological evaluation of the toxicity potential of the leaf extract of Stachytarpheta cayennensis in rats
- PMID: 33134130
- PMCID: PMC7588336
- DOI: 10.1016/j.jtcme.2019.05.001
Biochemical, hematological and histopathological evaluation of the toxicity potential of the leaf extract of Stachytarpheta cayennensis in rats
Abstract
Background and aim: The many pharmacological potentials of Stachytarpheta cayennensis (L.C. Rich) Vahl, especially in managing central nervous system disorders, hypertension, diabetes and infections, have made it a subject of abuse, necessitating the need to ascertain its safety. This study therefore investigated the toxic effects of the leaf extract of S. cayennensis in rats following acute and 28-day repeated doses in male and female rats.
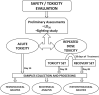
Experimental procedure: Acute and repeated dose studies were conducted in male and female groups of rats (135-150 g), using OECD 423 and 407 Tests guidelines respectively. Functional observational battery, and body weights were monitored. Blood samples were analysed for haematological and plasma biochemical indices. Organs (brain, kidneys and liver) specimen were collected and weighed. Kidney and liver specimen were subjected to histopathological analysis.
Results and conclusion: The LD50 of the extract was greater than 5000 mg/kg, p.o. (24 h) suggesting that the extract may be non-toxic. However, following single and repeated doses, the results revealed varying degree of significant (p < 0.05) changes in biochemical and heamatological indices, as well as in relative body weight and organ-body and organ-brain weight ratios. Also, histological assessment revealed evidence of liver and kidney toxicities and recovery was incomplete, as signs of toxicities were still evident after 21 days of recovery. Therefore, the extract is potentially harmful to vital organs with evidence of sex differential adverse effects and non-reversible forms of toxicity, especially with repeated usage, necessitating the need to avoid indiscriminate use.
Keywords: Acute toxicity; Organotoxicity; Repeated dose toxicity; Safety assessment; Stachytarpheta cayennesis.
© 2019 Center for Food and Biomolecules, National Taiwan University. Production and hosting by Elsevier Taiwan LLC.
Conflict of interest statement
None.
Figures





Similar articles
-
In Vitro and In Vivo Toxicological Evaluation of Avicennia africana P: Beauv. (Avicenniaceae) Leaf Extract in a Rat Model.J Toxicol. 2022 Nov 7;2022:3434383. doi: 10.1155/2022/3434383. eCollection 2022. J Toxicol. 2022. PMID: 36388260 Free PMC article.
-
Acute and sub-chronic toxicity studies of the aqueous extract from leaves of Cistus ladaniferus L. in mice and rats.J Ethnopharmacol. 2017 Sep 14;209:147-156. doi: 10.1016/j.jep.2017.07.029. Epub 2017 Jul 24. J Ethnopharmacol. 2017. PMID: 28750941
-
Safety assessment of the aqueous extract of the flowers of Nymphaea lotus Linn (Nymphaeaceae): Acute, neuro- and subchronic oral toxicity studies in albinos Wistar rats.J Complement Integr Med. 2017 Mar 24;14(2):/j/jcim.2017.14.issue-2/jcim-2016-0046/jcim-2016-0046.xml. doi: 10.1515/jcim-2016-0046. J Complement Integr Med. 2017. PMID: 28291734
-
Acute and sub-chronic toxicity studies of methanol extract of Trema orientalis (Linn) blume in albino wistar rats.Toxicol Rep. 2024 Sep 6;13:101723. doi: 10.1016/j.toxrep.2024.101723. eCollection 2024 Dec. Toxicol Rep. 2024. PMID: 39314231 Free PMC article.
-
Safety evaluation (acute and sub-acute studies) of the aqueous extract of the leaves of Myrianthus arboreus P. Beauv. (Cecropiaceae) in Wistar rats.J Ethnopharmacol. 2016 Dec 24;194:169-178. doi: 10.1016/j.jep.2016.08.052. Epub 2016 Aug 31. J Ethnopharmacol. 2016. PMID: 27592311
Cited by
-
Oral Toxicity and Hypotensive Influence of Sericin-Derived Oligopeptides (SDOs) from Yellow Silk Cocoons of Bombyx mori in Rodent Studies.Foods. 2024 Nov 1;13(21):3505. doi: 10.3390/foods13213505. Foods. 2024. PMID: 39517289 Free PMC article.
-
Acute and sub-acute toxicity assessment of methanolic stem bark extract of Khaya anthotheca (Meliaceae) in Wistar rats.Trop Med Health. 2025 Aug 29;53(1):120. doi: 10.1186/s41182-025-00721-9. Trop Med Health. 2025. PMID: 40883852 Free PMC article.
-
Safety evaluation of saffron extracts in early and established atherosclerotic New Zealand white rabbits.PLoS One. 2024 Jan 11;19(1):e0295212. doi: 10.1371/journal.pone.0295212. eCollection 2024. PLoS One. 2024. PMID: 38207245 Free PMC article.
-
Exploring the potential antimalarial properties, safety profile, and phytochemical composition of Mesua ferrea Linn.PLoS One. 2024 Dec 2;19(12):e0312047. doi: 10.1371/journal.pone.0312047. eCollection 2024. PLoS One. 2024. PMID: 39621736 Free PMC article.
-
Therapeutic Potential of Selected Medicinal Plants Against Carrageenan Induced Inflammation in Rats.Dose Response. 2021 Nov 27;19(4):15593258211058028. doi: 10.1177/15593258211058028. eCollection 2021 Oct-Dec. Dose Response. 2021. PMID: 34867126 Free PMC article.
References
-
- Ahmad I.A., Farrukh F., Owais M. Herbal medicines: prospects and constraints. In: Ahmad I.,F., Aqil, Owais M., editors. Modern Phytomedicine: Turning Medicinal Plants into Drugs. -VCH Verlag GmbH & Co.; Germany: 2006. pp. 59–78. - DOI
-
- Bandaranayake W.M. Quality control, screening, toxicity, and regulation of herbal drugs, In: Ahmad I.A.F., Owais M., editors. Modern Phytomedicine: Turning Medicinal Plants into Drugs. VCH Verlag GmbH & Co.; Germany: 2006. pp. 25–57.
-
- Hosseinzadeh S., Jafarikukhdan A., Hosseini A., Armand R. The application of medicinal plants in traditional and modern medicine: a review of. Thymus vulgaris Int J Clin Med. 2015;06(09):635–642. doi: 10.4236/ijcm.2015.69084. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
