The Role of Neurotransmitters in the Protection of Caenorhabditis Elegans for Salmonella Infection by Lactobacillus
- PMID: 33134188
- PMCID: PMC7550654
- DOI: 10.3389/fcimb.2020.554052
The Role of Neurotransmitters in the Protection of Caenorhabditis Elegans for Salmonella Infection by Lactobacillus
Abstract
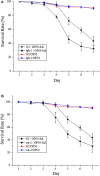
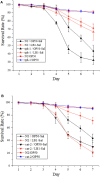
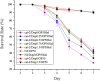
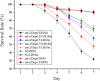
Salmonellosis is a common foodborne disease. We previously reported the protection of Caenorhabditis elegans from Salmonella Typhimurium DT104 infection by Lactobacillus zeae LB1. However, the mechanism is not fully understood. C. elegans exhibits behavior plasticity when presented with diverse pathogenic or commensal bacteria. Whether it can exert approach avoidance to S. Typhimurium through altering its neurological activity remains to be determined. In the current study, both the wild type and mutants defective in serotonin or dopamine production of C. elegans were used to investigate olfactory preference of the nematode to L. zeae LB1, DT104, and Escherichia coli OP50 by choice assays, and its resistance to DT104 infection and the protection offered by L. zeae LB1 using a life-span assay. The expression of target genes in C. elegans was also examined by real-time quantitative PCR. Results showed that pre-exposure to L. zeae LB1 did not elicit aversive olfactory behavior of the nematode toward DT104. Both mutants tph-1 and cat-2 succumbed faster than the wild type when infected with DT104. While pre-exposure to L. zeae LB1 significantly increased the survival of both the wild type and mutant tph-1, it provided no protection to mutant cat-2. Supplementation of dopamine resulted in both the resistance of mutant cat-2 to S. Typhimurium infection and the protection from L. zeae LB1 to the same mutant. Gene expression data also supported the observations in the life-span assay. These results suggest that both serotonin and dopamine play a positive role in the host defense of C. elegans to S. Typhimurium infection and that the L. zeae LB1 protection is not dependent on modifying olfactory preference of the nematode but mediated by dopamine that may have involved the regulation of p38-mitogen-activated protein kinase and insulin/insulin-like growth factor signaling pathways.
Keywords: Caenorhabditis elegans; Lactobacillus; Salmonella Typhimurium; neurotransmitters; olfactory behavior.
Copyright © 2020 Nie, Xie, and Her Majesty the Queen in Right of Canada, as represented by the Minister of Agriculture and Agri-Food Canada.
Figures

Similar articles
-
Lactobacillus Regulates Caenorhabditis elegans Cell Signaling to Combat Salmonella Infection.Front Immunol. 2021 Mar 8;12:653205. doi: 10.3389/fimmu.2021.653205. eCollection 2021. Front Immunol. 2021. PMID: 33763087 Free PMC article.
-
Cell Signaling of Caenorhabditis elegans in Response to Enterotoxigenic Escherichia coli Infection and Lactobacillus zeae Protection.Front Immunol. 2018 Sep 10;9:1745. doi: 10.3389/fimmu.2018.01745. eCollection 2018. Front Immunol. 2018. PMID: 30250464 Free PMC article.
-
Lactobacillus zeae protects Caenorhabditis elegans from enterotoxigenic Escherichia coli-caused death by inhibiting enterotoxin gene expression of the pathogen.PLoS One. 2014 Feb 18;9(2):e89004. doi: 10.1371/journal.pone.0089004. eCollection 2014. PLoS One. 2014. PMID: 24558463 Free PMC article.
-
Caenorhabditis elegans as an alternative model to study senescence of host defense and the prevention by immunonutrition.Adv Exp Med Biol. 2012;710:19-27. doi: 10.1007/978-1-4419-5638-5_3. Adv Exp Med Biol. 2012. PMID: 22127882 Review.
-
Caenorhabditis Elegans and Probiotics Interactions from a Prolongevity Perspective.Int J Mol Sci. 2019 Oct 10;20(20):5020. doi: 10.3390/ijms20205020. Int J Mol Sci. 2019. PMID: 31658751 Free PMC article. Review.
Cited by
-
Prolonged Lifespan, Improved Perception, and Enhanced Host Defense of Caenorhabditis elegans by Lactococcus cremoris subsp. cremoris.Microbiol Spectr. 2022 Jun 29;10(3):e0045421. doi: 10.1128/spectrum.00454-21. Epub 2022 May 16. Microbiol Spectr. 2022. PMID: 35575499 Free PMC article.
-
Bi-directional elucidation of Lactiplantibacillus plantarum (RTA 8) intervention on the pathophysiology of gut-brain axis during Salmonella brain infection.Gut Pathog. 2022 Mar 2;14(1):11. doi: 10.1186/s13099-022-00484-2. Gut Pathog. 2022. PMID: 35236424 Free PMC article.
-
Lactobacillus Regulates Caenorhabditis elegans Cell Signaling to Combat Salmonella Infection.Front Immunol. 2021 Mar 8;12:653205. doi: 10.3389/fimmu.2021.653205. eCollection 2021. Front Immunol. 2021. PMID: 33763087 Free PMC article.
References
-
- Chambers J. R., Gong J. (2011). The intestinal microbiota and its modulation for Salmonella control in chickens, Food Res. Int. 44, 3149–3159. 10.1016/j.foodres.2011.08.017 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

