Nano-Sized Iron Sulfide: Structure, Synthesis, Properties, and Biomedical Applications
- PMID: 33134265
- PMCID: PMC7512625
- DOI: 10.3389/fchem.2020.00818
Nano-Sized Iron Sulfide: Structure, Synthesis, Properties, and Biomedical Applications
Abstract
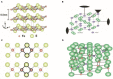
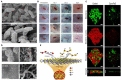
Nano-sized iron sulfides have attracted intense research interest due to the variety of their types, structures, and physicochemical properties. In particular, nano-sized iron sulfides exhibit enzyme-like activity by mimicking natural enzymes that depend on an iron-sulfur cluster as cofactor, extending their potential for applications in biomedicine. The present review principally summarizes the synthesis, properties and applications in biomedical fields, demonstrating that nano-sized iron sulfides have considerable potential for improving human health and quality of life.
Keywords: biomedical applications; enzyme-like activities; nano-sized iron sulfide; structure; synthesis.
Copyright © 2020 Yuan, Wang and Gao.
Figures









Similar articles
-
Biomedical applications of iron sulfide-based nanozymes.Front Chem. 2022 Aug 29;10:1000709. doi: 10.3389/fchem.2022.1000709. eCollection 2022. Front Chem. 2022. PMID: 36105309 Free PMC article. Review.
-
Preparation of Iron-Based Sulfides and Their Applications in Biomedical Fields.Biomimetics (Basel). 2023 Apr 24;8(2):177. doi: 10.3390/biomimetics8020177. Biomimetics (Basel). 2023. PMID: 37218763 Free PMC article. Review.
-
High-nuclearity sulfide-rich molybdenum[bond]iron[bond]sulfur clusters: reevaluation and extension.Inorg Chem. 2002 Jun 17;41(12):3191-201. doi: 10.1021/ic0201250. Inorg Chem. 2002. PMID: 12054998
-
Recent advances in metal sulfides: from controlled fabrication to electrocatalytic, photocatalytic and photoelectrochemical water splitting and beyond.Chem Soc Rev. 2019 Jul 29;48(15):4178-4280. doi: 10.1039/c8cs00664d. Chem Soc Rev. 2019. PMID: 31206105 Review.
-
A facile route to nano-sized nickel sulfide via thermolysis of nickel alkanethiolate.J Nanosci Nanotechnol. 2008 Sep;8(9):4873-6. doi: 10.1166/jnn.2008.ic57. J Nanosci Nanotechnol. 2008. PMID: 19049128
Cited by
-
Metal Sulfide Nanoparticles for Imaging and Phototherapeutic Applications.Molecules. 2023 Mar 10;28(6):2553. doi: 10.3390/molecules28062553. Molecules. 2023. PMID: 36985525 Free PMC article. Review.
-
Biomedical applications of iron sulfide-based nanozymes.Front Chem. 2022 Aug 29;10:1000709. doi: 10.3389/fchem.2022.1000709. eCollection 2022. Front Chem. 2022. PMID: 36105309 Free PMC article. Review.
-
Inorganic Fe-O and Fe-S oxidoreductases: paradigms for prebiotic chemistry and the evolution of enzymatic activity in biology.Front Chem. 2024 Feb 8;12:1349020. doi: 10.3389/fchem.2024.1349020. eCollection 2024. Front Chem. 2024. PMID: 38389729 Free PMC article. Review.
-
Photodynamic Therapeutic Effect of Nanostructured Metal Sulfide Photosensitizers on Cancer Treatment.Nanoscale Res Lett. 2022 Mar 8;17(1):33. doi: 10.1186/s11671-022-03674-8. Nanoscale Res Lett. 2022. PMID: 35258742 Free PMC article. Review.
-
Iron oxide nanozymes as versatile analytical tools: an overview of their application as detection technique.Bioanalysis. 2024 Dec-Dec;16(23-24):1261-1278. doi: 10.1080/17576180.2024.2415779. Epub 2024 Nov 26. Bioanalysis. 2024. PMID: 39589819 Review.
References
-
- Ahuja R., Sidhu A., Bala A. (2019). Synthesis and evaluation of iron(ii) sulfide aqua nanoparticles (FeS-NPs) against Fusarium verticillioides causing sheath rot and seed discoloration of rice. Eur. J. Plant Pathol. 155, 163–171. 10.1007/s10658-019-01758-3 - DOI
-
- Argueta-Figueroa L., Martinez-Alvarez O., Santos-Cruz J., Garcia-Contreras R., Acosta-Torres L. S., Fuente-Hernández J., et al. . (2017). Nanomaterials made of non-toxic metallic sulfides: a systematic review of their potential biomedical applications. Mater. Sci. Eng. C 76, 1305–1315. 10.1016/j.msec.2017.02.120 - DOI - PubMed
-
- Argueta-Figueroa L., Torres-Gómez N., García-Contreras R., Vilchis-Nestor A. R., Martínez-Alvarez O., Acosta-Torres L. S., et al. (2018). Hydrothermal synthesis of pyrrhotite (Fex−1S) nanoplates and their antibacterial, cytotoxic activity study. Progress Nat. Sci. 28, 447–455. 10.1016/j.pnsc.2018.06.003 - DOI
Publication types
LinkOut - more resources
Full Text Sources

