LncRNA MALAT1 Affects Mycoplasma pneumoniae Pneumonia via NF-κB Regulation
- PMID: 33134293
- PMCID: PMC7561720
- DOI: 10.3389/fcell.2020.563693
LncRNA MALAT1 Affects Mycoplasma pneumoniae Pneumonia via NF-κB Regulation
Abstract
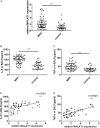
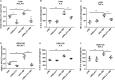
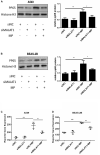
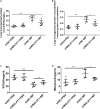
Our aim was to determine whether the long non-coding RNA (lncRNA) metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is involved in Mycoplasma pneumoniae pneumonia (MPP), and its possible mechanism of action. MALAT1 expression in the bronchoalveolar lavage fluid of 50 hospitalized children with MPP was compared to its expression in 30 children with intrabronchial foreign bodies. MALAT1 expression was higher in children with MPP, accompanied by increased inflammatory mediators interleukin 8 (IL-8) and tumor necrosis factor alpha (TNF-α), compared to the controls. In human airway epithelial cells infected with wild-type Mycoplasma pneumoniae (strain M129), MALAT1, IL-8, and TNF-α expression significantly increased, and increased expression of IL-8 and TNF-α could be suppressed by MALAT1 knockdown. Luciferase reporter gene assay and western blot showed that knockdown of MALAT1 reduced nuclear factor-κB (NF-κB) activation. In vivo, RNAi packaged with adenovirus (Adv) was nasally transfected into BALB/c mice to silence MALAT1, and an MP-infected mouse pneumonia model was prepared. The results demonstrated that the degree of pulmonary inflammatory injury, vascular permeability, secretion of inflammatory factors, and expression of phosphorylated p65 (pp65) in MP-infected mice were partly reversed after MALAT1 knockdown compared to those in the controls. In conclusion, MALAT1 is involved in the regulation of airway and pulmonary inflammation caused by MP infection via NF-κB regulation.
Keywords: Mycoplasma pneumoniae pneumonia; inflammation; lncRNA; metastasis associated lung adenocarcinoma transcript 1; nuclear factor-κB.
Copyright © 2020 Gu, Zhu, Zhou, Huang, Zhang, Zhao and Liu.
Figures


Similar articles
-
Toll-Like Receptor 2 Modulates Pulmonary Inflammation and TNF-α Release Mediated by Mycoplasma pneumoniae.Front Cell Infect Microbiol. 2022 Mar 17;12:824027. doi: 10.3389/fcimb.2022.824027. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35372108 Free PMC article.
-
Influence of lncRNA MALAT1 on septic lung injury in mice through p38 MAPK/p65 NF-κB pathway.Eur Rev Med Pharmacol Sci. 2019 Feb;23(3):1296-1304. doi: 10.26355/eurrev_201902_17025. Eur Rev Med Pharmacol Sci. 2019. PMID: 30779099
-
Attenuated lncRNA NKILA Enhances the Secretory Function of Airway Epithelial Cells Stimulated by Mycoplasma pneumoniae via NF-κB.Biomed Res Int. 2021 Mar 26;2021:6656298. doi: 10.1155/2021/6656298. eCollection 2021. Biomed Res Int. 2021. PMID: 33855076 Free PMC article.
-
Long Non-Coding RNA PACER Regulates Mycoplasma pneumoniae-induced Inflammatory Response through Interaction with NF-κB.Ann Clin Lab Sci. 2022 Jan;52(1):21-26. Ann Clin Lab Sci. 2022. PMID: 35181614
-
Ameliorative effects of Qingfei Tongluo formula on experimental mycoplasmal pneumonia in mice.J Nat Med. 2016 Apr;70(2):145-51. doi: 10.1007/s11418-015-0944-2. Epub 2015 Nov 20. J Nat Med. 2016. PMID: 26590157
Cited by
-
scRNA-Seq: First Atlas and Cellular Landscape of Lacrimal Sac: Implications in Primary Acquired Nasolacrimal Duct Obstruction Pathogenesis.Invest Ophthalmol Vis Sci. 2024 Mar 5;65(3):38. doi: 10.1167/iovs.65.3.38. Invest Ophthalmol Vis Sci. 2024. PMID: 38551583 Free PMC article.
-
Baicalin relieves Mycoplasma pneumoniae infection‑induced lung injury through regulating microRNA‑221 to inhibit the TLR4/NF‑κB signaling pathway.Mol Med Rep. 2021 Aug;24(2):571. doi: 10.3892/mmr.2021.12210. Epub 2021 Jun 10. Mol Med Rep. 2021. PMID: 34109422 Free PMC article.
-
Focus on long non-coding RNA MALAT1: Insights into acute and chronic lung diseases.Front Genet. 2022 Sep 16;13:1003964. doi: 10.3389/fgene.2022.1003964. eCollection 2022. Front Genet. 2022. PMID: 36186445 Free PMC article. Review.
-
Mechanistic studies of MALAT1 in respiratory diseases.Front Mol Biosci. 2022 Nov 7;9:1031861. doi: 10.3389/fmolb.2022.1031861. eCollection 2022. Front Mol Biosci. 2022. PMID: 36419933 Free PMC article. Review.
-
Toll-Like Receptor 2 Modulates Pulmonary Inflammation and TNF-α Release Mediated by Mycoplasma pneumoniae.Front Cell Infect Microbiol. 2022 Mar 17;12:824027. doi: 10.3389/fcimb.2022.824027. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35372108 Free PMC article.
References
LinkOut - more resources
Full Text Sources

