Circular RNAs: Functions and Clinical Significance in Cardiovascular Disease
- PMID: 33134301
- PMCID: PMC7550538
- DOI: 10.3389/fcell.2020.584051
Circular RNAs: Functions and Clinical Significance in Cardiovascular Disease
Abstract
Cardiovascular disease (CVD) causes high morbidity and mortality worldwide. Accumulating research has indicated the possible roles played by circular RNAs (circRNAs) in the pathogenesis of CVD. CircRNAs are non-coding RNAs with covalently closed loop structures. CircRNAs can function by acting as miRNA sponges, RNA binding protein sponges, mRNA transcriptional regulators and templates for protein translation. The specific characteristics of circRNAs such as high stability, abundant distribution, and tissue- and developmental stage-specific expression make them potential biomarkers for the diagnosis and prognosis of CVD. In this paper, we systematically summarized the current knowledge regarding the biogenesis, biological properties and the action mechanisms of circRNAs, elucidated the roles played by circRNAs in the pathogenesis of CVD, and explored the diagnostic potential of circRNAs in CVD. With in-depth studies, an increasing number of molecular mechanisms underlying the participation of circRNAs in CVD may be elucidated, and the application of circRNAs in the clinical diagnosis and prevention of CVD may eventually be realized.
Keywords: cardiovascular disease; circular RNAs; clinical application; diagnosis; pathogenesis.
Copyright © 2020 Zhang, Zhang, Wang, Zhao, Ding and Li.
Figures
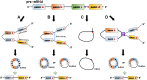

 ) to influence their functions. (C) CircRNAs can be the templates to encode proteins with the help of internal ribosome entry site (IRES
) to influence their functions. (C) CircRNAs can be the templates to encode proteins with the help of internal ribosome entry site (IRES ) elements or m6A-modification (brown pin
) elements or m6A-modification (brown pin ). (D) circRNAs with intronic sequences can regulate the expression of parental genes through binding to RNA polymerase II (Pol II). The circRNAs involved in CVD are listed in the blue box. The possible diagnostic biomarkers are displayed in the pink box. EcRNA, exonic circRNA; EIciRNA, exon–intron circRNA; CiRNA, circular intronic RNA.
). (D) circRNAs with intronic sequences can regulate the expression of parental genes through binding to RNA polymerase II (Pol II). The circRNAs involved in CVD are listed in the blue box. The possible diagnostic biomarkers are displayed in the pink box. EcRNA, exonic circRNA; EIciRNA, exon–intron circRNA; CiRNA, circular intronic RNA.Similar articles
-
Circular RNAs: A novel type of biomarker and genetic tools in cancer.Oncotarget. 2017 Jun 2;8(38):64551-64563. doi: 10.18632/oncotarget.18350. eCollection 2017 Sep 8. Oncotarget. 2017. PMID: 28969093 Free PMC article. Review.
-
Circular RNAs in Cardiovascular Disease: An Overview.Biomed Res Int. 2017;2017:5135781. doi: 10.1155/2017/5135781. Epub 2017 Jan 22. Biomed Res Int. 2017. PMID: 28210621 Free PMC article. Review.
-
Circular RNAs and Cardiovascular Regeneration.Front Cardiovasc Med. 2021 Apr 13;8:672600. doi: 10.3389/fcvm.2021.672600. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33928139 Free PMC article. Review.
-
Pathogenic mechanisms and the potential clinical value of circFoxo3 in cancers.Mol Ther Nucleic Acids. 2021 Jan 20;23:908-917. doi: 10.1016/j.omtn.2021.01.010. eCollection 2021 Mar 5. Mol Ther Nucleic Acids. 2021. PMID: 33614239 Free PMC article. Review.
-
Circular RNAs: biology and clinical significance of breast cancer.RNA Biol. 2023 Jan;20(1):859-874. doi: 10.1080/15476286.2023.2272468. Epub 2023 Oct 26. RNA Biol. 2023. PMID: 37882644 Free PMC article. Review.
Cited by
-
Current Knowledge of MicroRNAs (miRNAs) in Acute Coronary Syndrome (ACS): ST-Elevation Myocardial Infarction (STEMI).Life (Basel). 2021 Oct 8;11(10):1057. doi: 10.3390/life11101057. Life (Basel). 2021. PMID: 34685428 Free PMC article. Review.
-
Current Status of Functional Studies on Circular RNAs in Rheumatoid Arthritis and Their Potential Role as Diagnostic Biomarkers.J Inflamm Res. 2021 Mar 30;14:1185-1193. doi: 10.2147/JIR.S302846. eCollection 2021. J Inflamm Res. 2021. PMID: 33833541 Free PMC article. Review.
-
Involvement of circRNAs in the Development of Heart Failure.Int J Mol Sci. 2022 Nov 16;23(22):14129. doi: 10.3390/ijms232214129. Int J Mol Sci. 2022. PMID: 36430607 Free PMC article. Review.
-
Circ_0138959/miR-495-3p/TRAF6 axis regulates proliferation, wound healing and osteoblastic differentiation of periodontal ligament cells in periodontitis.J Dent Sci. 2022 Jul;17(3):1125-1134. doi: 10.1016/j.jds.2022.01.010. Epub 2022 Feb 15. J Dent Sci. 2022. PMID: 35784154 Free PMC article.
-
circ_0000045 promotes proliferation, migration, and invasion of head and neck squamous cell carcinomas via regulating HSP70 and MAPK pathway.BMC Cancer. 2022 Jul 20;22(1):799. doi: 10.1186/s12885-022-09880-y. BMC Cancer. 2022. PMID: 35854245 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

