Is There an Interplay Between the Functional Domains of IRAP?
- PMID: 33134302
- PMCID: PMC7550531
- DOI: 10.3389/fcell.2020.585237
Is There an Interplay Between the Functional Domains of IRAP?
Abstract
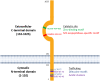
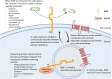
As a member of the M1 family of aminopeptidases, insulin regulated aminopeptidase (IRAP) is characterized by distinct binding motifs at the active site in the C-terminal domain that mediate the catalysis of peptide substrates. However, what makes IRAP unique in this family of enzymes is that it also possesses trafficking motifs at the N-terminal domain which regulate the movement of IRAP within different intracellular compartments. Research on the role of IRAP has focused predominantly on the C-terminus catalytic domain in different physiological and pathophysiological states ranging from pregnancy to memory loss. Many of these studies have utilized IRAP inhibitors, that bind competitively to the active site of IRAP, to explore the functional significance of its catalytic activity. However, it is unknown whether these inhibitors are able to access intracellular sites where IRAP is predominantly located in a basal state as the enzyme may need to be at the cell surface for the inhibitors to mediate their effects. This property of IRAP has often been overlooked. Interestingly, in some pathophysiological states, the distribution of IRAP is altered. This, together with the fact that IRAP possesses trafficking motifs, suggest the localization of IRAP may play an important role in defining its physiological or pathological functions and provide insights into the interplay between the two functional domains of the protein.
Keywords: Angiotensin IV; IRAP; IRAP inhibitors; M1 aminopeptidase; pathophysiology; trafficking.
Copyright © 2020 Vear, Gaspari, Thompson and Chai.
Figures
References
-
- Albiston A. L., Fernando R. N., Yeatman H. R., Burns P., Ng L., Daswani D., et al. (2010). Gene knockout of insulin-regulated aminopeptidase: loss of the specific binding site for angiotensin IV and age-related deficit in spatial memory. Neurobiol. Learn. Mem. 93 19–30. 10.1016/j.nlm.2009.07.011 - DOI - PubMed
-
- Albiston A. L., Pederson E. S., Burns P., Purcell B., Wright J. W., Harding J. W., et al. (2004). Attenuation of scopolamine-induced learning deficits by LVV-hemorphin-7 in rats in the passive avoidance and water maze paradigms. Behav. Brain Res. 154 239–243. 10.1016/j.bbr.2004.02.012 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

