Shortwave Infrared-Emitting Theranostics for Breast Cancer Therapy Response Monitoring
- PMID: 33134314
- PMCID: PMC7575924
- DOI: 10.3389/fmolb.2020.569415
Shortwave Infrared-Emitting Theranostics for Breast Cancer Therapy Response Monitoring
Abstract
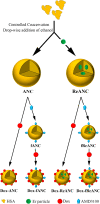
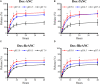
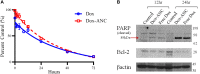
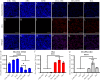
Therapeutic drug monitoring (TDM) in cancer, while imperative, has been challenging due to inter-patient variability in drug pharmacokinetics. Additionally, most pharmacokinetic monitoring is done by assessments of the drugs in plasma, which is not an accurate gauge for drug concentrations in target tumor tissue. There exists a critical need for therapy monitoring tools that can provide real-time feedback on drug efficacy at target site to enable alteration in treatment regimens early during cancer therapy. Here, we report on theranostic optical imaging probes based on shortwave infrared (SWIR)-emitting rare earth-doped nanoparticles encapsulated with human serum albumin (abbreviated as ReANCs) that have demonstrated superior surveillance capability for detecting micro-lesions at depths of 1 cm in a mouse model of breast cancer metastasis. Most notably, ReANCs previously deployed for detection of multi-organ metastases resolved bone lesions earlier than contrast-enhanced magnetic resonance imaging (MRI). We engineered tumor-targeted ReANCs carrying a therapeutic payload as a potential theranostic for evaluating drug efficacy at the tumor site. In vitro results demonstrated efficacy of ReANCs carrying doxorubicin (Dox), providing sustained release of Dox while maintaining cytotoxic effects comparable to free Dox. Significantly, in a murine model of breast cancer lung metastasis, we demonstrated the ability for therapy monitoring based on measurements of SWIR fluorescence from tumor-targeted ReANCs. These findings correlated with a reduction in lung metastatic burden as quantified via MRI-based volumetric analysis over the course of four weeks. Future studies will address the potential of this novel class of theranostics as a preclinical pharmacological screening tool.
Keywords: NIR-II bioimaging; nanotechnology; rare earths; shortwave infrared imaging; theranostic; tumor therapy monitoring.
Copyright © 2020 Shah, Gonda, Pemmaraju, Subash, Bobadilla Mendez, Berger, Zhao, He, Riman, Tan, Pierce, Moghe and Ganapathy.
Figures


Similar articles
-
Early Detection of Myeloid-Derived Suppressor Cells in the Lung Pre-Metastatic Niche by Shortwave Infrared Nanoprobes.Pharmaceutics. 2024 Apr 17;16(4):549. doi: 10.3390/pharmaceutics16040549. Pharmaceutics. 2024. PMID: 38675210 Free PMC article.
-
Shortwave infrared emitting multicolored nanoprobes for biomarker-specific cancer imaging in vivo.BMC Cancer. 2020 Nov 10;20(1):1082. doi: 10.1186/s12885-020-07604-8. BMC Cancer. 2020. PMID: 33172421 Free PMC article.
-
Surveillance nanotechnology for multi-organ cancer metastases.Nat Biomed Eng. 2017;1:993-1003. doi: 10.1038/s41551-017-0167-9. Epub 2017 Dec 12. Nat Biomed Eng. 2017. PMID: 29531851 Free PMC article.
-
CXCR-4 Targeted, Short Wave Infrared (SWIR) Emitting Nanoprobes for Enhanced Deep Tissue Imaging and Micrometastatic Cancer Lesion Detection.Small. 2015 Dec 16;11(47):6347-57. doi: 10.1002/smll.201502202. Epub 2015 Oct 30. Small. 2015. PMID: 26514367 Free PMC article.
-
Nanotechnology for the theranostic opportunity of breast cancer lung metastasis: recent advancements and future challenges.Front Bioeng Biotechnol. 2024 May 31;12:1410017. doi: 10.3389/fbioe.2024.1410017. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38882636 Free PMC article. Review.
Cited by
-
Current State of Breast Cancer Diagnosis, Treatment, and Theranostics.Pharmaceutics. 2021 May 14;13(5):723. doi: 10.3390/pharmaceutics13050723. Pharmaceutics. 2021. PMID: 34069059 Free PMC article. Review.
-
Recent Advances in Fluorescence Imaging of Traumatic Brain Injury in Animal Models.Front Mol Biosci. 2021 May 26;8:660993. doi: 10.3389/fmolb.2021.660993. eCollection 2021. Front Mol Biosci. 2021. PMID: 34124151 Free PMC article. Review.
-
Web-Based Application for Biomedical Image Registry, Analysis, and Translation (BiRAT).Tomography. 2022 May 30;8(3):1453-1462. doi: 10.3390/tomography8030117. Tomography. 2022. PMID: 35736865 Free PMC article.
-
Shortwave-Infrared-Emitting Nanoprobes for CD8 Targeting and In Vivo Imaging of Cytotoxic T Cells in Breast Cancer.Adv Nanobiomed Res. 2024 Feb;4(2):2300092. doi: 10.1002/anbr.202300092. Epub 2023 Dec 5. Adv Nanobiomed Res. 2024. PMID: 39554690 Free PMC article.
-
Early Detection of Myeloid-Derived Suppressor Cells in the Lung Pre-Metastatic Niche by Shortwave Infrared Nanoprobes.Pharmaceutics. 2024 Apr 17;16(4):549. doi: 10.3390/pharmaceutics16040549. Pharmaceutics. 2024. PMID: 38675210 Free PMC article.
References
-
- Andhariya N., Chudasama B., Mehta R. V., Upadhyay R. V. (2010). Biodegradable thermoresponsive polymeric magnetic nanoparticles: a new drug delivery platform for doxorubicin. J. Nanopart. Res. 13 1677–1688. 10.1007/s11051-010-9921-9926 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

