Direct Oral Anticoagulants vs. Vitamin-K Antagonists in the Elderly With Atrial Fibrillation: A Systematic Review Comparing Benefits and Harms Between Observational Studies and Randomized Controlled Trials
- PMID: 33134323
- PMCID: PMC7511536
- DOI: 10.3389/fcvm.2020.00132
Direct Oral Anticoagulants vs. Vitamin-K Antagonists in the Elderly With Atrial Fibrillation: A Systematic Review Comparing Benefits and Harms Between Observational Studies and Randomized Controlled Trials
Abstract
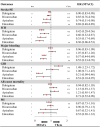
Background: The publication of high-quality observational studies (OSs) has fueled reassessment of the treatment effects of direct oral anticoagulants (DOACs) in the elderly with atrial fibrillation (AF). Methods: The MEDLINE, EMBASE, and Cochrane Library databases were systematically searched (through July 1, 2019) for eligible OSs and randomized controlled trials (RCTs) that reported effectiveness outcomes [stroke or systemic embolism (SE)] or safety outcomes [intracranial hemorrhage (ICH), major bleeding, gastrointestinal bleeding (GIB), myocardial infarction (MI), and all-cause mortality] for DOACs and vitamin-K antagonists (VKAs) in elderly AF patients. A random-effects model was applied to calculate adjusted hazard ratios (HRs) for OSs and relative risks (RRs) for RCTs. Interaction analyses and the ratio of HR (RHR) were used to assess and compare OSs and RCTs. Results: A total of 32 studies involving 547,419 patients were included. No significant difference in treatment effect estimates was found between 27 OSs and 5 RCTs [P interaction > 0.05 for each and all 95% confidence interval (CI) of RHR crossed 1.0]. Compared with VKAs, DOACs significantly reduced risk for stroke/SE (OSs, HR: 0.87, 95% CI: 0.81-0.94; RCT, RR: 0.82, 95% CI: 0.67-0.96), and ICH (OSs: 0.47 [0.37-0.57]; RCTs: 0.47 [0.31-0.63]), without increasing risk for GIB (OSs: 1.21 [0.98-1.43]; RCTs: 1.34 [0.91-1.77]), and all-cause mortality (OSs: 1.01 [0.92-1.11]; RCTs: 0.94 [0.87-1.00]). Among OSs, DOACs significantly decreased risk for major bleeding (0.87 [0.77-0.98]) and MI (0.89 [0.79-0.99]). It was found that dabigatran, but not other DOACs, significantly increased risk for GIB (1.48 [1.23-1.72]). Conclusions: DOACs were demonstrated to be more effective and safer than VKAs in elderly AF patients, whereas dabigatran users had a 48% increase in risk for GIB.
Keywords: bleeding; dabigatran; embolism; real-world study; stroke; warfarin.
Copyright © 2020 Shen, Wu, Wang, Kong, Zhang, Wang, Gu and Chen.
Figures


Similar articles
-
Comparison of effectiveness and safety of direct oral anticoagulants versus vitamin-k antagonists in elderly patients with atrial fibrillation: a systematic review and cost-effectiveness analysis protocol.Ann Transl Med. 2020 Mar;8(6):391. doi: 10.21037/atm.2020.02.109. Ann Transl Med. 2020. PMID: 32355835 Free PMC article.
-
Direct Oral Anticoagulants vs. Warfarin in Latin American Patients With Atrial Fibrillation: Evidence From Four post-hoc Analyses of Randomized Clinical Trials.Front Cardiovasc Med. 2022 Mar 4;9:841341. doi: 10.3389/fcvm.2022.841341. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35310968 Free PMC article.
-
Direct versus conventional anticoagulants for treatment of cancer associated thrombosis: a pooled and interaction analysis between observational studies and randomized clinical trials.Ann Transl Med. 2020 Feb;8(4):95. doi: 10.21037/atm.2019.12.152. Ann Transl Med. 2020. PMID: 32175388 Free PMC article.
-
Direct Oral Anticoagulants vs. Vitamin K Antagonists in Atrial Fibrillation Patients at Risk of Falling: A Meta-Analysis.Front Cardiovasc Med. 2022 May 9;9:833329. doi: 10.3389/fcvm.2022.833329. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35615562 Free PMC article.
-
Direct oral anticoagulants versus warfarin in nonvalvular atrial fibrillation patients with prior gastrointestinal bleeding: a network meta-analysis of real-world data.Eur J Clin Pharmacol. 2022 Jul;78(7):1057-1067. doi: 10.1007/s00228-022-03300-7. Epub 2022 Mar 16. Eur J Clin Pharmacol. 2022. PMID: 35296907 Review.
Cited by
-
Real world data of anticoagulant treatment in non-valvular atrial fibrillation across renal function status.Sci Rep. 2022 Apr 12;12(1):6123. doi: 10.1038/s41598-022-10164-5. Sci Rep. 2022. PMID: 35414001 Free PMC article.
-
Current Use of Oral Anticoagulation Therapy in Elderly Patients with Atrial Fibrillation: Results from an Italian Multicenter Prospective Study-The ISNEP Study.J Pers Med. 2022 Aug 31;12(9):1419. doi: 10.3390/jpm12091419. J Pers Med. 2022. PMID: 36143204 Free PMC article.
-
Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials: a meta-epidemiological study.Cochrane Database Syst Rev. 2024 Jan 4;1(1):MR000034. doi: 10.1002/14651858.MR000034.pub3. Cochrane Database Syst Rev. 2024. PMID: 38174786 Free PMC article.
-
Direct Oral Anticoagulants versus Vitamin K Antagonists in Patients Aged 80 Years and Older.Int J Environ Res Public Health. 2021 Apr 22;18(9):4443. doi: 10.3390/ijerph18094443. Int J Environ Res Public Health. 2021. PMID: 33922331 Free PMC article.
-
Direct Oral Anticoagulants in Older and Frail Patients with Atrial Fibrillation: A Decade of Experience.Drugs Aging. 2024 Sep;41(9):725-740. doi: 10.1007/s40266-024-01138-5. Epub 2024 Aug 14. Drugs Aging. 2024. PMID: 39141209 Free PMC article. Review.
References
-
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. (2010) 137:263–72. 10.1378/chest.09-1584 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

