Effect of anticoagulant adjustment on prothrombin time test using two different PT reagents in patients with elevated hematocrit
- PMID: 33134464
- PMCID: PMC7585133
- DOI: 10.1016/j.plabm.2020.e00177
Effect of anticoagulant adjustment on prothrombin time test using two different PT reagents in patients with elevated hematocrit
Abstract
The recommendations for adjustment of citrate volume in sample tubes with high hematocrit (Ht) are based on indirect studies of underfilled tubes or artificially constructed Ht values. The aim of this study was to evaluate the effect of citrate volume adjustment in sample tubes from patients with hematocrit >55% using two different prothrombin time (PT) tests.
Methods: Paired citrate-adjusted and unadjusted blood specimens were obtained from 181 patients from the pulmonary hypertension ambulatory with high Ht values and on warfarin therapy. The samples were tested using recombinant human tissue factor (RTF) and reagents extracted from rabbit brain (HS Plus). The results are expressed as the international normalized ratio (INR). The correlation and percent change (% change) between sample pairs were calculated.
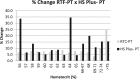
Results: INR-RTF results from adjusted and unadjusted citrate blood specimens showed a strong correlation (R2 = 0.8226, p < 0.0001). The INR median was 2.25 (95% CI 2.10 to 2.41) for citrate-adjusted samples and was 2.22 (95% CI 2.06 to 2.38) for citrate-unadjusted samples. For samples with Ht >62%, the % change between sample pairs was >10%. Results using HS Plus showed a moderate correlation between citrate-adjusted and unadjusted samples (R2 = 0.4267, p < 0.0001). The INR median was 2.51 (95% CI 2.35 to 2.68) for citrate-adjusted samples and 3.45 (95% CI 3.11 to 3.80) for citrate-unadjusted samples. For samples with Ht>55%, the % change between sample pairs was higher than 10%.
Conclusion: Our data demonstrate that in patients with polycythemia on warfarin therapy, INR-RTF does not require anticoagulant adjustment for assessment of samples with Ht <62%.
Keywords: Citrate adjustment; Hematocrit; Prothrombin time; Thromboplastins.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
References
-
- Pai S.H., Michalaros K. Effect of sample volume on coagulation tests. Lab. Med. 1990;21(6):371–373. doi: 10.1093/labmed/21.6.371. - DOI
-
- Who Expert Committee on Biological Standardization . World Health Organization Technical Report Series; 1999. Guidelines for thromboplastins and plasma used to monitor oral anticoagulant therapy; p. 889. - PubMed
-
- Fairweather R.B., Ansell J., van den Besselaar A.M., Brandt J.T., Bussey H.I., Poller L., Triplett D.A., White R.H. College of American Pathologists Conference XXXI on laboratory monitoring of anticoagulant therapy: laboratory monitoring of oral anticoagulant therapy. Arch. Pathol. Lab Med. 1998;122(9):768–781. - PubMed
LinkOut - more resources
Full Text Sources