Pooled testing conserves SARS-CoV-2 laboratory resources and improves test turn-around time: experience on the Kenyan Coast
- PMID: 33134555
- PMCID: PMC7590893
- DOI: 10.12688/wellcomeopenres.16113.2
Pooled testing conserves SARS-CoV-2 laboratory resources and improves test turn-around time: experience on the Kenyan Coast
Abstract
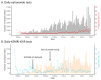
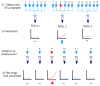
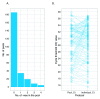
Background. International recommendations for the control of the coronavirus disease 2019 (COVID-19) pandemic emphasize the central role of laboratory testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent, at scale. The availability of testing reagents, laboratory equipment and qualified staff are important bottlenecks to achieving this. Elsewhere, pooled testing (i.e. combining multiple samples in the same reaction) has been suggested to increase testing capacities in the pandemic period. Methods. We discuss our experience with SARS-CoV-2 pooled testing using real-time reverse transcription polymerase chain reaction (RT-PCR) on the Kenyan Coast. Results. In mid-May, 2020, our RT-PCR testing capacity for SARS-CoV-2 was improved by ~100% as a result of adoption of a six-sample pooled testing strategy. This was accompanied with a concomitant saving of ~50% of SARS-CoV-2 laboratory test kits at both the RNA extraction and RT-PCR stages. However, pooled testing came with a slight decline of test sensitivity. The RT-PCR cycle threshold value (ΔCt) was ~1.59 higher for samples tested in pools compared to samples tested singly. Conclusions. Pooled testing is a useful strategy to increase SARS-CoV-2 laboratory testing capacity especially in low-income settings.
Keywords: COVID-19; Kenya; Kilifi; Pooled testing; SARS-CoV-2.
Copyright: © 2021 Agoti CN et al.
Conflict of interest statement
No competing interests were disclosed.
Figures
References
-
- MoH: First Case of Coronavirus Disease Confirmed in Kenya. MoH, Editor. Ministry of Health: Press release.2020;1–3. Reference Source
-
- MoH: COVID-19 Outbreak in Kenya. In Daily Situation Report 122, E.O. Center, Editor. MoH: MoH website.2020;1–4. Reference Source
-
- Nanyingi M: The evolution of the COVID-19 pandemic in Kenya. T.R.S.o.T.M.a. Hygiene, Editor.2020. Reference Source
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

