Criteria pollutant impacts of volatile chemical products informed by near-field modeling
- PMID: 33134558
- PMCID: PMC7592713
- DOI: 10.1038/s41893-020-00614-1
Criteria pollutant impacts of volatile chemical products informed by near-field modeling
Abstract
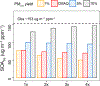
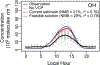
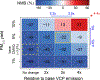
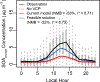
Consumer, industrial, and commercial product usage is a source of exposure to potentially hazardous chemicals. In addition, cleaning agents, personal care products, coatings, and other volatile chemical products (VCPs), evaporate and react in the atmosphere producing secondary pollutants. Here, we show high air emissions from VCP usage (≥ 14 kg person-1 yr-1, at least 1.7× higher than current operational estimates) are supported by multiple estimation methods and constraints imposed by ambient levels of ozone, hydroxyl radical (OH) reactivity, and the organic component of fine particulate matter (PM2.5) in Pasadena, California. A near-field model, which estimates human chemical exposure during or in the vicinity of product use, indicates these high air emissions are consistent with organic product usage up to ~75 kg person-1 yr-1, and inhalation of consumer products could be a non-negligible exposure pathway. After constraining the PM2.5 yield to 5% by mass, VCPs produce ~41% of the photochemical organic PM2.5 (1.1 ± 0.3 μg m-3) and ~17% of maximum daily 8-hr average ozone (9 ± 2 ppb) in summer Los Angeles. Therefore, both toxicity and ambient criteria pollutant formation should be considered when organic substituents are developed for VCPs in pursuit of safer and sustainable products and cleaner air.
Figures



References
-
- McDonald BC et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 359, 760–764, (2018). - PubMed
-
- Trantallidi M et al. Ephect III: Health risk assessment of exposure to household consumer products. Sci. Total Environ 536, 903–913, (2015). - PubMed
-
- Elberling J et al. Mucosal symptoms elicited by fragrance products in a population-based sample in relation to atopy and bronchial hyper-reactivity. Clin. Exp. Allergy 35, 75–81, (2005). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
