Nature of Metal-Drug Bond in Some Antitumor Active Complexes of Coinage Metal Ions
- PMID: 33134660
- PMCID: PMC7594011
- DOI: 10.1021/acsomega.0c01471
Nature of Metal-Drug Bond in Some Antitumor Active Complexes of Coinage Metal Ions
Abstract
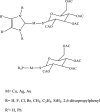
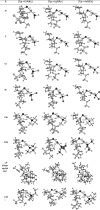
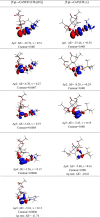
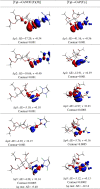
N-Heterocyclic carbene and phosphine can be labeled as solid σ-donor ligands and can contribute to stable complexes. In addition, the constructed complex can accommodate a wide variety of applications, such as pharmaceutical products. In the light of this, a theoretical analysis was carried out on the existence of metal-drug interactions of group 11 metal ions in coordination with symmetrical unsaturated N-heterocyclic carbenes [NHC(R)(R')] and monodentate phosphine (PR3). The R substitutes on N atoms in NHC and phosphines are identical, and R' substitutes are located on two noncarbenic carbon atoms (C4 and C5) in the heterocycle complexes. All complexes are in general formula, [Tgt → ML] {where M = Cu(I), Ag(I), Au(I), Tgt = 2,3,4,6-tetra-O-acetyl-1-thio-β-d-glucopyranoside, L= [NHC(R)(R')], and PR3; R = F, Cl, Br, H, CH3, C2H5, SiH3, 2,6-diisopropylphenyl; R' = H and Ph} at the PBE-D3/def2-TZVP level of theory. Findings show greater tolerance for the release of drugs in the presence of Ag(I) metal ions than the other metal ions studied here. Applying natural bond orbital (NBO), atoms in molecules (AIMs), energy decomposition analysis (EDA), and extended transition-state natural orbital for chemical valence (ETS-NOCV) analysis have been researched in order to ascertain the nature of M ← S and M ← C (M ← P) bonds in the complexes. Results have shown that σ donation from S to M atoms in [Tgt → MPR3] complexes is better and the π acceptor is weaker than the corresponding [Tgt → MNHC(R)(R')] complexes.
© 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Petz W.; Frenking G.. Carbodiphosphoranes and Related Ligands. In Transition Metal Complexes of Neutral η1 -Carbon Ligands; Springer-Verlag: Berlin, 2010; Vol. 30, pp 49–92.
-
- Zhang X. F.; Sun M. J.; Cao Z. X. Theoretical study on interactions of N-heterocyclic carbene with the bare first-row transition metals. Theor. Chem. Acc. 2016, 135, 163–174. 10.1007/s00214-016-1922-9. - DOI
-
- Heinemann C.; Müller T.; Apeloig Y.; Schwarz H. On the Question of Stability, Conjugation, and “Aromaticity” in imidazole-2-ylidenes and their Silicon analogs. J. Am. Chem. Soc. 1996, 118, 2023–2038. 10.1021/ja9523294. - DOI
- Boehme C.; Frenking G. Electronic structure of stable carbenes, silylenes, and germylenes. J. Am. Chem. Soc. 1996, 118, 2039–2046. 10.1021/ja9527075. - DOI
- Hillier A. C.; Sommer W. J.; Yong B. S.; Petersen J. L.; Cavallo L.; Nolan S. P. A Combined experimental and theoretical study examining the binding of N-Heterocyclic carbenes (NHC) to the Cp*RuCl (Cp* = η5-C5Me5) moiety: Insight into stereoelectronic differences between unsaturated and saturated NHC ligands. Organometallics 2003, 22, 4322–4326. 10.1021/om034016k. - DOI
- Dröge T.; Glorius F. The measure of all rings-N–Heterocyclic carbenes. Angew. Chem., Int. Ed. 2010, 49, 6940–6952. 10.1002/anie.201001865. - DOI - PubMed
-
- Petz W.; Neumüller B.; Klein S.; Frenking G. Syntheses and crystal structures of [Hg{C(PPh3)2}2][Hg2I6] and [Cu{C(PPh3)2}2]I and comparative theoretical study of carbene complexes [M(NHC)2] with carbone complexes [M{C(PH3)2}2] (M = Cu+, Ag+, Au+, Zn2+, Cd2+, Hg2+). Organometallics 2011, 30, 3330–3339. 10.1021/om200145c. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

