Intermolecular Interaction between Heavy Crude Oils and Surfactants during Surfactant-Steam Flooding Process
- PMID: 33134701
- PMCID: PMC7594125
- DOI: 10.1021/acsomega.0c00193
Intermolecular Interaction between Heavy Crude Oils and Surfactants during Surfactant-Steam Flooding Process
Abstract
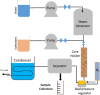
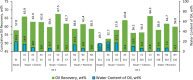
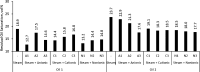
The objective of this study is to investigate the intermolecular interactions between the surfactants and the fractions of heavy crude oils. Two possible interactions were considered; polar and ionic interactions for two heavy crude oil-surfactant systems, and 20 surfactant-steam flooding tests were conducted on these crudes by testing nine surfactants (three anionic, three cationic, and three nonionic) with different tail lengths and charged head groups. The performance differences observed in each core flood were discussed through the additional analyses. To explain polar interactions, the pseudo blends of crude oil fractions (fractionation of saturates, aromatics, resins, and asphaltenes) were exposed to the surfactant solutions under vapor and liquid water conditions and their mutual interactions were visualized under an optical microscope. To explain ionic interactions, the charges on asphaltene surfaces were analyzed by zeta potential measurements before and after core flood tests on both the produced and the residual oil asphaltenes. The addition of surfactants improved the oil recovery when compared to steam injection alone. However, different oil recoveries were obtained with different surfactants. Further analyses showed that asphaltenes are key and the interaction of asphaltenes with other crude oil fractions or surfactants determines the success of surfactant-steam processes. The polar interactions favor the emulsion formation more; hence, if the polar interactions are more dominant than the ion interactions in the overall crude oil-surfactant system, the surfactant flooding process into heavy oil reservoir became more successful.
© 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Green D.W.; Willhite P.G.. Enhanced Oil Recovery, First Edition. Richardson, TX: Henry L. Doherty Memorial Fund of AIME; 1998; pp. 239–287
-
- Henderson J.H.; Taber J.J.. Residual Oil Recovery Process Using Water Containing a Surfactant. In: Google Patents US3,199,586A1965.
-
- Uren L. C.; Fahmy E. Factors Influencing the Recovery of Petroleum from Unconsolidated Sands by Waterflooding. Trans. Am. Inst. Min., Metall., Pet. Eng., Soc. Min. Eng. AIME 1927, 77, 318–335. 10.2118/927318-G. - DOI
-
- Myers D.Surfactant Science and Technology, Third edition John Wiley & Sons; 2005; pp. 3–55.
LinkOut - more resources
Full Text Sources

