Chemoresistive Room-Temperature Sensing of Ammonia Using Zeolite Imidazole Framework and Reduced Graphene Oxide (ZIF-67/rGO) Composite
- PMID: 33134712
- PMCID: PMC7594151
- DOI: 10.1021/acsomega.0c03981
Chemoresistive Room-Temperature Sensing of Ammonia Using Zeolite Imidazole Framework and Reduced Graphene Oxide (ZIF-67/rGO) Composite
Abstract
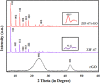
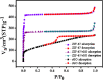
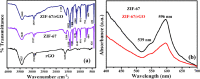
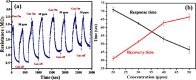
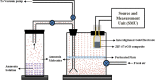
The present work demonstrates the application of a composite of the zeolite imidazole framework (ZIF-67) and reduced graphene oxide (rGO), synthesized via a simple hydrothermal route for the sensitive sensing of ammonia. The successful synthesis of ZIF-67 and rGO composite was confirmed with structural and spectroscopic characterizations. A porous structure and a high surface area (1080 m2 g-1) of the composite indicate its suitability as a gas sensing material. The composite material was coated as a thin film onto interdigitated gold electrodes. The sensor displays a change in its chemoresistive property (i.e., resistance) in the presence of ammonia (NH3) gas. A sensor response of 1.22 ± 0.02 [standard deviation (sd)] is measured for 20 ppm of NH3, while it shows a value of 4.77 ± 0.15 (sd) for 50 ppm of NH3. The fabricated sensor is reproducible and offers a stable response, while also providing tolerance against humidity and some other volatile compounds. The average response and recovery times of the sensor, for 50 ppm NH3 concentration, are found to be 46.5 ± 2.12 (sd) and 66.5 ± 2.12 (sd) s, respectively. The limit of detection of the sensor was found to be 74 ppb.
© 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
A Room-Temperature Surface Acoustic Wave Ammonia Sensor Based on rGO/DPP2T-TT Composite Films.Sensors (Basel). 2022 Jul 14;22(14):5280. doi: 10.3390/s22145280. Sensors (Basel). 2022. PMID: 35890960 Free PMC article.
-
NH3 Sensor Based on rGO-PANI Composite with Improved Sensitivity.Sensors (Basel). 2021 Jul 21;21(15):4947. doi: 10.3390/s21154947. Sensors (Basel). 2021. PMID: 34372184 Free PMC article.
-
Biphenyl-rGO composite room temperature gas sensor for enhanced amine sensing.Chemosphere. 2024 Mar;351:141244. doi: 10.1016/j.chemosphere.2024.141244. Epub 2024 Jan 17. Chemosphere. 2024. PMID: 38242515
-
A synergistic approach to enhance sensitivity and selectivity of room temperature operable ammonia gas sensor with humidity assistance using RGO/WO3nanocomposite.Nanotechnology. 2023 Nov 22;35(6). doi: 10.1088/1361-6528/ad090a. Nanotechnology. 2023. PMID: 37918025
-
Boosting room-temperature ppb-level NO2 sensing over reduced graphene oxide by co-decoration of α-Fe2O3 and SnO2 nanocrystals.J Colloid Interface Sci. 2022 Apr 15;612:689-700. doi: 10.1016/j.jcis.2022.01.009. Epub 2022 Jan 6. J Colloid Interface Sci. 2022. PMID: 35030345
Cited by
-
Recent trends in gas sensing via carbon nanomaterials: outlook and challenges.Nanoscale Adv. 2021 Oct 28;3(23):6514-6544. doi: 10.1039/d1na00707f. eCollection 2021 Nov 24. Nanoscale Adv. 2021. PMID: 36132656 Free PMC article. Review.
-
Multilayered Mesoporous Composite Nanostructures for Highly Sensitive Label-Free Quantification of Cardiac Troponin-I.Biosensors (Basel). 2022 May 14;12(5):337. doi: 10.3390/bios12050337. Biosensors (Basel). 2022. PMID: 35624638 Free PMC article.
-
Fabrication of a Fully Printed Ammonia Gas Sensor Based on ZnO/rGO Using Ultraviolet-Ozone Treatment.Sensors (Basel). 2024 Mar 6;24(5):1691. doi: 10.3390/s24051691. Sensors (Basel). 2024. PMID: 38475227 Free PMC article.
-
A humidity-resistant and room temperature carbon soot@ZIF-67 composite sensor for acetone vapour detection.Nanoscale Adv. 2023 Feb 16;5(7):1956-1969. doi: 10.1039/d3na00050h. eCollection 2023 Mar 28. Nanoscale Adv. 2023. PMID: 36998651 Free PMC article.
-
Zeolitic imidazolate framework as humidity-resistant solid state-chemiresistive gas sensors: A review.Heliyon. 2023 Nov 15;9(11):e22329. doi: 10.1016/j.heliyon.2023.e22329. eCollection 2023 Nov. Heliyon. 2023. PMID: 38034700 Free PMC article. Review.
References
-
- Toxic FAQ Sheet of Ammonia; Agency for Toxic Substances and Disease Registry (ATSDR), 2004; CAS 7664-41-7.
-
- National Research Council . Acute Exposure Guideline Levels for Selected Airborne Chemicals; National Academies Press: Washington, DC, 2003; Vol. 3.
-
- Timmer B.; Olthuis W.; van den Berg A. Ammonia sensors and their applications—A Review. Sens. Actuators, B 2005, 107, 666–677. 10.1016/j.snb.2004.11.054. - DOI
-
- Tai H.; Duan Z.; He Z.; Li X.; Xu J.; Liu B.; Jiang Y. Enhanced ammonia response of Ti3C2Txnanosheets supported by TiO2 nanoparticles at room temperature. Sens. Actuators, B 2019, 298, 126874.10.1016/j.snb.2019.126874. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

