Second-line treatments and outcomes for immune thrombocytopenia: A retrospective study with electronic health records
- PMID: 33134779
- PMCID: PMC7590333
- DOI: 10.1002/rth2.12423
Second-line treatments and outcomes for immune thrombocytopenia: A retrospective study with electronic health records
Abstract
Background: Second-line treatment for immune thrombocytopenia (ITP) is not well reported for patients treated in real-world clinical settings.
Objective: The purpose of this study was to compare outcomes of four second-line treatments for ITP.
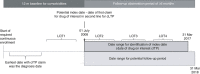
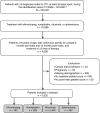
Patients/methods: Included adult patients had at least two medical records containing ITP diagnoses and second-line eltrombopag, romiplostim, rituximab, or splenectomy. Date of treatment initiation or splenectomy was set as index date, between July 1, 2008, and March 31, 2017. Patients had first-line corticosteroid or intravenous immune globulin treatment and continuous database activity from 6 months before to 12 months after index. Patient characteristics, treatment patterns, platelet counts, bleeding-related episodes (BREs), and thrombotic events (TEs) were compared by second-line treatment cohort.
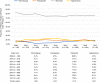
Results: The sample included 3332 patients (mean age, 60.5 years; 52.3% female): eltrombopag (5.8%), romiplostim (9.9%), rituximab (73.3%), and splenectomy (11.0%). Patients having splenectomy were younger, more likely female and commercially insured, and less likely to require a third line of treatment than medical regimen cohorts. Proportions of patients having treatment-free (≥180 days with no second-line index or rescue agent) periods varied significantly (P = .01) by regimen: 33% for eltrombopag, 23% for romiplostim, 26% for rituximab, and 17% for splenectomy. All regimens significantly improved platelet counts, while TE and BRE rates differed significantly (P = .03 and P = .01, respectively) when all treatment groups were compared.
Conclusions: Over an average 7-year follow-up, all second-line regimens improved platelet counts, but eltrombopag yielded the highest proportion of patients with completely treatment-free periods of at least 180 days.
Keywords: eltrombopag; rituximab; romiplostim; splenectomy; thrombocytopenia.
© 2020 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis (ISTH).
Figures

Similar articles
-
Early initiation of second-line therapy in primary immune thrombocytopenia: insights from real-world evidence.Ann Hematol. 2023 Aug;102(8):2051-2058. doi: 10.1007/s00277-023-05289-0. Epub 2023 Jun 10. Ann Hematol. 2023. PMID: 37300567 Free PMC article.
-
Treatment patterns and outcomes of second-line rituximab and thrombopoietin receptor agonists in adult immune thrombocytopenia: A Canadian retrospective cohort study.Thromb Res. 2022 Dec;220:5-11. doi: 10.1016/j.thromres.2022.09.021. Epub 2022 Sep 29. Thromb Res. 2022. PMID: 36257098
-
Cost effectiveness of romiplostim for the treatment of chronic immune thrombocytopenia in Ireland.Appl Health Econ Health Policy. 2013 Oct;11(5):457-69. doi: 10.1007/s40258-013-0044-y. Appl Health Econ Health Policy. 2013. PMID: 23857462 Free PMC article.
-
[Diagnostic approach and treatment of immune thrombocytopenia in adults].Acta Med Croatica. 2013 Mar;67(1):3-11. Acta Med Croatica. 2013. PMID: 24279250 Review. Croatian.
-
Romiplostim in adults with newly diagnosed or persistent immune thrombocytopenia.Expert Rev Hematol. 2020 Dec;13(12):1319-1332. doi: 10.1080/17474086.2020.1850253. Epub 2020 Nov 30. Expert Rev Hematol. 2020. PMID: 33249935 Review.
Cited by
-
Consensus guidelines for the management of adult immune thrombocytopenia in Australia and New Zealand.Med J Aust. 2022 Jan 17;216(1):43-52. doi: 10.5694/mja2.51284. Epub 2021 Oct 10. Med J Aust. 2022. PMID: 34628650 Free PMC article.
-
Primary Immune Thrombocytopenia: Novel Insights into Pathophysiology and Disease Management.J Clin Med. 2021 Feb 16;10(4):789. doi: 10.3390/jcm10040789. J Clin Med. 2021. PMID: 33669423 Free PMC article. Review.
-
Bisphosphonate use for glucocorticoid-induced osteoporosis in older patients with immune thrombocytopenia: a clinical perspective.Ann Hematol. 2023 Jul;102(7):1645-1656. doi: 10.1007/s00277-023-05266-7. Epub 2023 May 12. Ann Hematol. 2023. PMID: 37171596 Free PMC article. Review.
-
Thrombopoietin receptor agonist discontinuation rates and reasons among patients with immune thrombocytopenia: a study of administrative claims linked with medical chart review.Ann Hematol. 2022 Sep;101(9):1915-1924. doi: 10.1007/s00277-022-04888-7. Epub 2022 Jul 18. Ann Hematol. 2022. PMID: 35849155
-
Therapeutic Outcomes of High Dose-Dexamethasone versus Prednisolone + Azathioprine, Rituximab, Eltrombopag, and Romiplostim Strategies in Persistent, Chronic, Refractory, and Relapsed Immune Thrombocytopenia Patients.Pharmaceuticals (Basel). 2023 Aug 29;16(9):1215. doi: 10.3390/ph16091215. Pharmaceuticals (Basel). 2023. PMID: 37765023 Free PMC article.
References
-
- Weycker D, Hanau A, Hatfield M, Wu H, Sharma A, Bensink ME, et al. Primary immune thrombocytopenia in US clinical practice: incidence and healthcare burden in the first 12 months following diagnosis. J Med Econ. 2020;23(2):184–92. - PubMed
-
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr, Crowther MA. American Society of Hematology. The American Society of Hematology 2011 evidence‐based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–207. - PubMed
-
- Raj AB. Immune thrombocytopenia: pathogenesis and treatment approaches. J Hematol Transfus. 2017;5(1):1056.
LinkOut - more resources
Full Text Sources

