Systematic Review and STARD Scoring of Renal Cell Carcinoma Circulating Diagnostic Biomarker Manuscripts
- PMID: 33134830
- PMCID: PMC7583155
- DOI: 10.1093/jncics/pkaa050
Systematic Review and STARD Scoring of Renal Cell Carcinoma Circulating Diagnostic Biomarker Manuscripts
Abstract
Background: No validated molecular biomarkers exist to help guide diagnosis of renal cell carcinoma (RCC) patients. We seek to evaluate the quality of published RCC circulating diagnostic biomarker manuscripts using the Standards for Reporting of Diagnostic Accuracy Studies (STARD) guidelines.
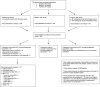
Methods: The phrase "(renal cell carcinoma OR renal cancer OR kidney cancer OR kidney carcinoma) AND circulating AND (biomarkers OR cell free DNA OR tumor DNA OR methylated cell free DNA OR methylated tumor DNA)" was searched in Embase, MEDLINE, and PubMed in March 2018. Relevant manuscripts were scored using 41 STARD subcriteria for a maximal score of 26 points. All tests of statistical significance were 2 sided.
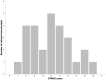
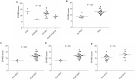
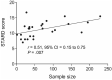
Results: The search identified 535 publications: 27 manuscripts of primary research were analyzed. The median STARD score was 11.5 (range = 7-16.75). All manuscripts had appropriate abstracts, introductions, and distribution of alternative diagnoses. None of the manuscripts stated how indeterminant data were handled or if adverse events occurred from performing the index test or reference standard. Statistically significantly higher STARD scores were present in manuscripts reporting receiver operator characteristic curves (P < .001), larger sample sizes (P = .007), and after release of the original STARD statement (P = .005).
Conclusions: Most RCC circulating diagnostic biomarker manuscripts poorly adhere to the STARD guidelines. Future studies adhering to STARD guidelines may address this unmet need.
© The Author(s) 2020. Published by Oxford University Press.
Figures
Similar articles
-
Systematic review and REMARK scoring of renal cell carcinoma prognostic circulating biomarker manuscripts.PLoS One. 2019 Oct 22;14(10):e0222359. doi: 10.1371/journal.pone.0222359. eCollection 2019. PLoS One. 2019. PMID: 31639128 Free PMC article.
-
Assessment of Standards for Reporting of Diagnostic Accuracy (STARD) 2015 guideline adherence in medical imaging diagnostic accuracy studies published in 2023.J Clin Epidemiol. 2025 Mar;179:111654. doi: 10.1016/j.jclinepi.2024.111654. Epub 2024 Dec 27. J Clin Epidemiol. 2025. PMID: 39733974
-
Adherence to STARD guidelines in miRNA diagnostic accuracy studies for hepatocellular carcinoma: a systematic review.BMC Med Res Methodol. 2025 Jun 6;25(1):155. doi: 10.1186/s12874-025-02610-5. BMC Med Res Methodol. 2025. PMID: 40481407 Free PMC article.
-
Emergency Ultrasound Literature and Adherence to Standards for Reporting of Diagnostic Accuracy Criteria.J Emerg Med. 2020 Apr;58(4):636-646. doi: 10.1016/j.jemermed.2019.09.029. Epub 2019 Nov 7. J Emerg Med. 2020. PMID: 31708317 Free PMC article.
-
Diagnostic DNA Methylation Biomarkers for Renal Cell Carcinoma: A Systematic Review.Eur Urol Oncol. 2021 Apr;4(2):215-226. doi: 10.1016/j.euo.2019.07.011. Epub 2019 Aug 9. Eur Urol Oncol. 2021. PMID: 31402218
References
-
- Battaglia M, Lucarelli G. The role of renal surgery in the era of targeted therapy: the urologist's perspective. Urologia. 2015;82(3):137–138. doi:10.5301/uro.5000105. - PubMed
-
- Crispen PL, Breau RH, Allmer C, et al.Lymph node dissection at the time of radical nephrectomy for high-risk clear cell renal cell carcinoma: indications and recommendations for surgical templates. Eur Urol. 2011;59(1):18–23. doi:10.1016/j.eururo.2010.08.042. - PubMed
-
- AJCC Cancer Staging Manual. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC... . Published 2010. Accessed February 21, 2019.
-
- Patard JJ, Escudier B. Urological cancer: towards rational post-nephrectomy follow-up guidelines in RCC. Nat Rev Clin Oncol. 2015;12(3):131–132. doi: 10.1038/nrclinonc.2015.17. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
