An Adaptive Control Scheme for Interleukin-2 Therapy
- PMID: 33134893
- PMCID: PMC7588844
- DOI: 10.1016/j.isci.2020.101663
An Adaptive Control Scheme for Interleukin-2 Therapy
Abstract
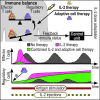
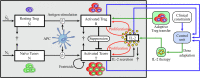
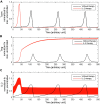
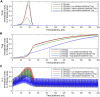
Regulatory T cells (Treg) are suppressor cells that control self-reactive and excessive effector conventional T helper cell (Tconv) responses. Breakdown of the balance between Tregs and Tconvs is a hallmark of autoimmune and inflammatory diseases. Interleukin-2 (IL-2) is a growth factor for both populations and subtle leverage to restore the healthy immune balance in IL-2 therapy. By using a mechanistic mathematical model, we introduced an adaptive control strategy to design the minimal therapeutic IL-2 dosage required to increase and stabilize Treg population and restrict inflammatory response. This adaptive protocol allows for dose adjustments based on the feedback of the immune kinetics of the patient. Our simulation results showed that a minimal Treg population was required to restrict the transient side effect of IL-2 injections on the effector Tconv response. In silico results suggested that a combination of IL-2 and adoptive Treg transfer therapies can limit this side effect.
Keywords: Biological Sciences; Immunology; Mathematical Bioscience.
© 2020 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
TCR signaling by conventional CD4+ T cells is required for optimal maintenance of peripheral regulatory T cell numbers.Immun Inflamm Dis. 2016 Mar 24;4(2):148-154. doi: 10.1002/iid3.100. eCollection 2016 Jun. Immun Inflamm Dis. 2016. PMID: 27891224 Free PMC article.
-
Protocol of the adaptive study of IL-2 dose frequency on regulatory T cells in type 1 diabetes (DILfrequency): a mechanistic, non-randomised, repeat dose, open-label, response-adaptive study.BMJ Open. 2015 Dec 8;5(12):e009799. doi: 10.1136/bmjopen-2015-009799. BMJ Open. 2015. PMID: 26646829 Free PMC article. Clinical Trial.
-
4-1BB Signaling in Conventional T Cells Drives IL-2 Production That Overcomes CD4+CD25+FoxP3+ T Regulatory Cell Suppression.PLoS One. 2016 Apr 6;11(4):e0153088. doi: 10.1371/journal.pone.0153088. eCollection 2016. PLoS One. 2016. PMID: 27049955 Free PMC article.
-
The role of regulatory T Cells in autoimmune orchitis.Andrologia. 2018 Dec;50(11):e13092. doi: 10.1111/and.13092. Andrologia. 2018. PMID: 30569653 Review.
-
[Treg/Th17 balance and immunology of schistosome infection: a review].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018 Oct 17;30(5):588-591. doi: 10.16250/j.32.1374.2018001. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2018. PMID: 30567041 Review. Chinese.
Cited by
-
Exploring the constituent mechanisms of hepatitis: a dynamical systems approach.Math Med Biol. 2023 Mar 13;40(1):24-48. doi: 10.1093/imammb/dqac013. Math Med Biol. 2023. PMID: 36197900 Free PMC article.
-
Dynamically modeling the effective range of IL-2 dosage in the treatment of systemic lupus erythematosus.iScience. 2022 Aug 11;25(9):104911. doi: 10.1016/j.isci.2022.104911. eCollection 2022 Sep 16. iScience. 2022. PMID: 36060072 Free PMC article.
-
Interleukin-2 superkines by computational design.Proc Natl Acad Sci U S A. 2022 Mar 22;119(12):e2117401119. doi: 10.1073/pnas.2117401119. Epub 2022 Mar 16. Proc Natl Acad Sci U S A. 2022. PMID: 35294290 Free PMC article.
References
-
- Bahremand S., Ko H.S., Balouchzadeh R., Lee H.F., Park S., Kwon G. Neural network-based model predictive control for type 1 diabetic rats on artificial pancreas system. Med. Biol. Eng. Comput. 2019;57:177–191. - PubMed
-
- Fontes F.A., Pereira F.L. Model predictive control of impulsive dynamical systems. IFAC Proc. Volumes. 2012;45:305–310.
LinkOut - more resources
Full Text Sources
Other Literature Sources

