Volatile Isoflurane in Critically Ill Coronavirus Disease 2019 Patients-A Case Series and Systematic Review
- PMID: 33134946
- PMCID: PMC7587445
- DOI: 10.1097/CCE.0000000000000256
Volatile Isoflurane in Critically Ill Coronavirus Disease 2019 Patients-A Case Series and Systematic Review
Abstract
Objectives: The ongoing coronavirus pandemic is challenging, especially in severely affected patients who require intubation and sedation. Although the potential benefits of sedation with volatile anesthetics in coronavirus disease 2019 patients are currently being discussed, the use of isoflurane in patients with coronavirus disease 2019-induced acute respiratory distress syndrome has not yet been reported.
Design: We performed a retrospective analysis of critically ill patients with hypoxemic respiratory failure requiring mechanical ventilation.
Setting: The study was conducted with patients admitted between April 4 and May 15, 2020 to our ICU.
Patients: We included five patients who were previously diagnosed with severe acute respiratory syndrome coronavirus 2 infection.
Intervention: Even with high doses of several IV sedatives, the targeted level of sedation could not be achieved. Therefore, the sedation regimen was switched to inhalational isoflurane. Clinical data were recorded using a patient data management system. We recorded demographical data, laboratory results, ventilation variables, sedative dosages, sedation level, prone positioning, duration of volatile sedation and outcomes.
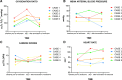
Measurements & main results: Mean age (four men, one women) was 53.0 (± 12.7) years. The mean duration of isoflurane sedation was 103.2 (± 66.2) hours. Our data demonstrate a substantial improvement in the oxygenation ratio when using isoflurane sedation. Deep sedation as assessed by the Richmond Agitation and Sedation Scale was rapidly and closely controlled in all patients, and the subsequent discontinuation of IV sedation was possible within the first 30 minutes. No adverse events were detected.
Conclusions: Our findings demonstrate the feasibility of isoflurane sedation in five patients suffering from severe coronavirus disease 2019 infection. Volatile isoflurane was able to achieve the required deep sedation and reduced the need for IV sedation.
Keywords: acute respiratory distress syndrome; coronavirus disease 2019; critical care; deep sedation; severe acute respiratory syndrome coronavirus 2; volatile sedation.
Copyright © 2020 The Authors. Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine.
Conflict of interest statement
The authors have disclosed that they do not have any potential conflicts of interest.
Figures
References
-
- Bösel J, Purrucker JC, Nowak F, et al. Volatile isoflurane sedation in cerebrovascular intensive care patients using AnaConDa(®): Effects on cerebral oxygenation, circulation, and pressure. Intensive Care Med. 2012; 38:1955–1964 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

