Updated perspectives on vascular cell specification and pluripotent stem cell-derived vascular organoids for studying vasculopathies
- PMID: 33135070
- PMCID: PMC8752356
- DOI: 10.1093/cvr/cvaa313
Updated perspectives on vascular cell specification and pluripotent stem cell-derived vascular organoids for studying vasculopathies
Abstract
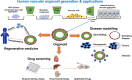
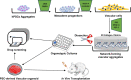
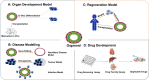
Vasculopathy is a pathological process occurring in the blood vessel wall, which could affect the haemostasis and physiological functions of all the vital tissues/organs and is one of the main underlying causes for a variety of human diseases including cardiovascular diseases. Current pharmacological interventions aiming to either delay or stop progression of vasculopathies are suboptimal, thus searching novel, targeted, risk-reducing therapeutic agents, or vascular grafts with full regenerative potential for patients with vascular abnormalities are urgently needed. Since first reported, pluripotent stem cells (PSCs), particularly human-induced PSCs, have open new avenue in all research disciplines including cardiovascular regenerative medicine and disease remodelling. Assisting with recent technological breakthroughs in tissue engineering, in vitro construction of tissue organoid made a tremendous stride in the past decade. In this review, we provide an update of the main signal pathways involved in vascular cell differentiation from human PSCs and an extensive overview of PSC-derived tissue organoids, highlighting the most recent discoveries in the field of blood vessel organoids as well as vascularization of other complex tissue organoids, with the aim of discussing the key cellular and molecular players in generating vascular organoids.
Keywords: Blood vessel; Embryonic stem cell; Induced pluripotent stem cell; Organoid vascularization; Pluripotent stem cell; Tissue-engineered vascular graft; Vascular disease; Vascular endothelial cell; Vascular organoid; Vascular smooth muscle cell; Vasculopathy.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2020. For permissions, please email: journals.permissions@oup.com.
Figures




Similar articles
-
Blood flow and stem cells in vascular disease.Cardiovasc Res. 2013 Jul 15;99(2):251-9. doi: 10.1093/cvr/cvt061. Epub 2013 Mar 20. Cardiovasc Res. 2013. PMID: 23519267 Review.
-
Induction of vascular progenitor cells from endothelial cells stimulates coronary collateral growth.Circ Res. 2012 Jan 20;110(2):241-52. doi: 10.1161/CIRCRESAHA.111.250126. Epub 2011 Nov 17. Circ Res. 2012. PMID: 22095729 Free PMC article.
-
New models to study vascular mural cell embryonic origin: implications in vascular diseases.Cardiovasc Res. 2018 Mar 15;114(4):481-491. doi: 10.1093/cvr/cvy005. Cardiovasc Res. 2018. PMID: 29385541 Review.
-
Differentiation and Application of Induced Pluripotent Stem Cell-Derived Vascular Smooth Muscle Cells.Arterioscler Thromb Vasc Biol. 2017 Nov;37(11):2026-2037. doi: 10.1161/ATVBAHA.117.309196. Epub 2017 Aug 31. Arterioscler Thromb Vasc Biol. 2017. PMID: 28860223 Review.
-
Novel concepts for the role of smooth muscle cells in vascular disease: towards a new smooth muscle cell classification.Cardiovasc Res. 2018 Mar 15;114(4):477-480. doi: 10.1093/cvr/cvy031. Cardiovasc Res. 2018. PMID: 29408963 No abstract available.
Cited by
-
Biological Materials for Tissue-Engineered Vascular Grafts: Overview of Recent Advancements.Biomolecules. 2023 Sep 14;13(9):1389. doi: 10.3390/biom13091389. Biomolecules. 2023. PMID: 37759789 Free PMC article. Review.
-
Human mini-blood-brain barrier models for biomedical neuroscience research: a review.Biomater Res. 2022 Dec 16;26(1):82. doi: 10.1186/s40824-022-00332-z. Biomater Res. 2022. PMID: 36527159 Free PMC article. Review.
-
Vascularization of kidney organoids: different strategies and perspectives.Front Urol. 2024 May 21;4:1355042. doi: 10.3389/fruro.2024.1355042. eCollection 2024. Front Urol. 2024. PMID: 40777107 Free PMC article. Review.
-
Bioengineering methods for vascularizing organoids.Cell Rep Methods. 2024 Jun 17;4(6):100779. doi: 10.1016/j.crmeth.2024.100779. Epub 2024 May 16. Cell Rep Methods. 2024. PMID: 38759654 Free PMC article. Review.
-
hPSC-derived lung organoids: Potential opportunities and challenges.Heliyon. 2023 Feb 4;9(2):e13498. doi: 10.1016/j.heliyon.2023.e13498. eCollection 2023 Feb. Heliyon. 2023. PMID: 36814627 Free PMC article. Review.
References
-
- Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, Estep K, Cercy K, Murray CJL, Forouzanfar MH.. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol 2016;15:913–924. - PubMed
-
- Bonaca MP, Creager MA.. Pharmacological treatment and current management of peripheral artery disease. Circ Res 2015;116:1579–1598. - PubMed
-
- Berliner JA, Navab M, Fogelman AM, Frank JS, Demer LL, Edwards PA, Watson AD, Lusis AJ.. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation 1995;91:2488–2496. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

