Investigation of acidic free-glycans in urine and their alteration in cancer
- PMID: 33135073
- PMCID: PMC8091460
- DOI: 10.1093/glycob/cwaa100
Investigation of acidic free-glycans in urine and their alteration in cancer
Abstract
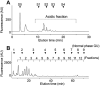
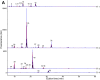
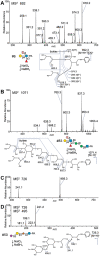
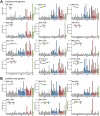
Alterations to glycans in cancer patients have been used to identify novel tumor biomarkers. Most of these studies have focused on protein glycosylation but less attention has been paid to free-glycans. Here, we analyzed acidic free-glycans in the urine of cancer patients to identify novel tumor marker candidates. Specifically, urine samples were collected from patients with gastric cancer, pancreatic cancer and cholangiocarcinoma as well as normal controls. The free-glycans were extracted from creatinine-adjusted urine and fluorescently labeled with 2-aminopyridine. Initially, we performed profiling of urinary free-glycans by high-performance liquid chromatography and mass spectrometry with enzymatic and chemical degradation. More than 100 glycans, including novel structures, were identified. The chromatographic peaks suggested some of these glycans were present at elevated levels in cancer patients. To verify cancer-associated alterations, we compared the glycan levels between cancer patients and normal controls by selected reaction monitoring. Representative structures of glycans with elevated levels in cancer patients included the following: small glycans related to sialyllactose; sialyl Lewis X; lactose- and N-acetyllactosamine (LacNAc) type-II-core glycans with LacNAc (type-I or II)-extensions and modifications of α1,3/4-fucose and/or 6-sulfate on the Glc/GlcNAc; free-N-glycans containing sialylation or β1,6-branch of 6-sulfo Lewis X; novel NeuAcα2-3Galβ1-4(+/-Fucα1-3) Xylα1-3Glc glycans. Our results provide further insight into urinary free-glycans and suggest the potential utility of these compounds as tumor markers.
Keywords: HPLC; SRM; cancer; free glycan; urine.
© The Author(s) 2020. Published by Oxford University Press.
Figures




References
-
- Arnold JN, Saldova R, Galligan MC, Murphy TB, Mimura-Kimura Y, Telford JE, Godwin AK, Rudd PM. 2011. Novel glycan biomarkers for the detection of lung cancer. J Proteome Res. 10:1755–1764. - PubMed
-
- Biskup K, Braicu EI, Sehouli J, Fotopoulou C, Tauber R, Berger M, Blanchard V. 2013. Serum glycome profiling: A biomarker for diagnosis of ovarian cancer. J Proteome Res. 12:4056–4063. - PubMed
-
- Björndal H, Lundblad A. 1970. Structure of two urinary oligosaccharides characteristic of blood group O(H)- and B-secretors. Biochim Biophys Acta. 201:434–437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

