ABC-F translation factors: from antibiotic resistance to immune response
- PMID: 33135152
- PMCID: PMC8609467
- DOI: 10.1002/1873-3468.13984
ABC-F translation factors: from antibiotic resistance to immune response
Abstract
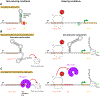
Energy-dependent translational throttle A (EttA) from Escherichia coli is a paradigmatic ABC-F protein that controls the first step in polypeptide elongation on the ribosome according to the cellular energy status. Biochemical and structural studies have established that ABC-F proteins generally function as translation factors that modulate the conformation of the peptidyl transferase center upon binding to the ribosomal tRNA exit site. These factors, present in both prokaryotes and eukaryotes but not in archaea, use related molecular mechanisms to modulate protein synthesis for heterogenous purposes, ranging from antibiotic resistance and rescue of stalled ribosomes to modulation of the mammalian immune response. Here, we review the canonical studies characterizing the phylogeny, regulation, ribosome interactions, and mechanisms of action of the bacterial ABC-F proteins, and discuss the implications of these studies for the molecular function of eukaryotic ABC-F proteins, including the three human family members.
Keywords: ABC ATPase; ABC-F protein family; antibiotic resistance; immune response; infection; mRNA translation; protein synthesis.
© 2020 Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures

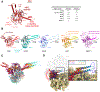



References
-
- Pakotiprapha D, Samuels M, Shen K, Hu JH & Jeruzalmi D (2012) Structure and mechanism of the UvrA-UvrB DNA damage sensor. Nature structural & molecular biology 19, 291–8. - PubMed
-
- Hopfner K-P, Karcher A, Shin DS, Craig L, Arthur LM, Carney JP & Tainer JA (2000) Structural Biology of Rad50 ATPase. Cell 101, 789–800. - PubMed
-
- Goosen N & Moolenaar GF (2001) Role of ATP hydrolysis by UvrA and UvrB during nucleotide excision repair. Research in Microbiology 152, 401–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

