Opposing JAK-STAT and Wnt signaling gradients define a stem cell domain by regulating differentiation at two borders
- PMID: 33135631
- PMCID: PMC7695452
- DOI: 10.7554/eLife.61204
Opposing JAK-STAT and Wnt signaling gradients define a stem cell domain by regulating differentiation at two borders
Abstract
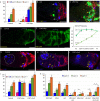
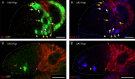
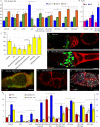
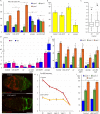
Many adult stem cell communities are maintained by population asymmetry, where stochastic behaviors of multiple individual cells collectively result in a balance between stem cell division and differentiation. We investigated how this is achieved for Drosophila Follicle Stem Cells (FSCs) by spatially-restricted niche signals. FSCs produce transit-amplifying Follicle Cells (FCs) from their posterior face and quiescent Escort Cells (ECs) to their anterior. We show that JAK-STAT pathway activity, which declines from posterior to anterior, dictates the pattern of divisions over the FSC domain, promotes more posterior FSC locations and conversion to FCs, while opposing EC production. Wnt pathway activity declines from the anterior, promotes anterior FSC locations and EC production, and opposes FC production. The pathways combine to define a stem cell domain through concerted effects on FSC differentiation to ECs and FCs at either end of opposing signaling gradients, and impose a pattern of proliferation that matches derivative production.
Keywords: D. melanogaster; Follicle Stem Cells; JAK-STAT; Stem Cells; Wnt; developmental biology; niche; regenerative medicine; signaling; stem cells.
Plain language summary
Adult organisms contain a variety of cells that are routinely replaced using adult stem cells which can generate the cells of a specific tissue. These stem cells are often clustered into small groups, where combinations of chemical signals from nearby cells can encourage each stem cell to divide or ‘differentiate’ into another type of cell. These different signals must somehow balance stem cell division and differentiation to maintain the size and shape of the community. The ovary of an adult fruit fly contains a group of adult stem cells called follicle stem cells, or FSCs for short. FSCs support the continual production of eggs by supplying two types of cell from opposite faces of the stem cell cluster: dividing follicle cells emerge from the back of the cluster and guide late egg development, while non-dividing escort cells come from the front and guide early egg development. Two of the signals that control FSCs are graded over the cluster. JAK-STAT signaling is strongest in the follicle cell territory and gradually declines towards the front, while Wnt signaling is strongest in escort cells and absent from early follicle cells. However, it was unclear how the gradients of these two signals maintain the FSC population and control the formation of follicle and escort cells. To answer this question, Melamed and Kalderon used genetic engineering to modify the strength of these two signals. The experiments measured how this affected the rate at which FSCs divide and are converted into follicle or escort cells. Melamed and Kalderon found that the strength of JAK-STAT signaling dictated division rates, which may explain why the rate cells divide varies across the FSC cluster and escort cells do not divide at all. JAK-STAT signaling also stimulated FSCs to become follicle cells and opposed their conversion to escort cells. Conversely, stronger Wnt signaling favored the production of escort cells and inhibited FSCs from transitioning to follicle cells. This suggests that the relative strength of these two opposing signals helps maintain thecorrect number of FSCs while also balancing the formation of follicle and escort cells. JAK-STAT, Wnt and other signals guide the development of many organisms, including humans, and have also been linked to cancer. Therefore, the principles and mechanisms uncovered may apply to other types of stem cells. Furthermore, this work highlights genetic changes that can allow a mutant stem cell to amplify and take over an entire stem cell community, which may play a role in cancer and other illnesses.
© 2020, Melamed and Kalderon.
Conflict of interest statement
DM, DK No competing interests declared
Figures

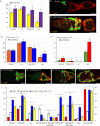
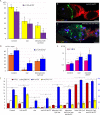
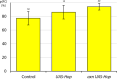
Similar articles
-
Spatial regulation of Drosophila ovarian Follicle Stem Cell division rates and cell cycle transitions.PLoS Genet. 2023 Sep 25;19(9):e1010965. doi: 10.1371/journal.pgen.1010965. eCollection 2023 Sep. PLoS Genet. 2023. PMID: 37747936 Free PMC article.
-
Regulation of somatic stem cell and niche precursor fates and proliferation of by Wnt, JAK-STAT, Hedgehog and Hippo/Yorkie pathways during Drosophila pupal ovary development resembles the signaling framework organizing adult stem cell behavior.bioRxiv [Preprint]. 2025 May 13:2025.05.08.652870. doi: 10.1101/2025.05.08.652870. bioRxiv. 2025. PMID: 40463270 Free PMC article. Preprint.
-
Single-cell expression profile of Drosophila ovarian follicle stem cells illuminates spatial differentiation in the germarium.BMC Biol. 2023 Jun 20;21(1):143. doi: 10.1186/s12915-023-01636-9. BMC Biol. 2023. PMID: 37340484 Free PMC article.
-
Drosophila Jak/STAT Signaling: Regulation and Relevance in Human Cancer and Metastasis.Int J Mol Sci. 2018 Dec 14;19(12):4056. doi: 10.3390/ijms19124056. Int J Mol Sci. 2018. PMID: 30558204 Free PMC article. Review.
-
Organogenesis and tumorigenesis: insight from the JAK/STAT pathway in the Drosophila eye.Dev Dyn. 2010 Oct;239(10):2522-33. doi: 10.1002/dvdy.22394. Dev Dyn. 2010. PMID: 20737505 Free PMC article. Review.
Cited by
-
Mechanism of piR-1245/PIWI-like protein-2 regulating Janus kinase-2/signal transducer and activator of transcription-3/vascular endothelial growth factor signaling pathway in retinal neovascularization.Neural Regen Res. 2023 May;18(5):1132-1138. doi: 10.4103/1673-5374.355819. Neural Regen Res. 2023. PMID: 36255003 Free PMC article.
-
Protocol for evaluating autophagy using LysoTracker staining in the epithelial follicle stem cells of the Drosophila ovary.STAR Protoc. 2021 Jun 11;2(2):100592. doi: 10.1016/j.xpro.2021.100592. eCollection 2021 Jun 18. STAR Protoc. 2021. PMID: 34169286 Free PMC article.
-
Sequential events during the quiescence to proliferation transition establish patterns of follicle cell differentiation in the Drosophila ovary.Biol Open. 2023 Jan 1;12(1):bio059625. doi: 10.1242/bio.059625. Epub 2023 Jan 12. Biol Open. 2023. PMID: 36524613 Free PMC article.
-
Distinct roles of Bendless in regulating FSC niche competition and daughter cell differentiation.Development. 2021 Nov 15;148(22):dev199630. doi: 10.1242/dev.199630. Epub 2021 Nov 22. Development. 2021. PMID: 35020878 Free PMC article.
-
Extracellular spreading of Wingless is required for Drosophila oogenesis.PLoS Genet. 2021 Apr 2;17(4):e1009469. doi: 10.1371/journal.pgen.1009469. eCollection 2021 Apr. PLoS Genet. 2021. PMID: 33798197 Free PMC article.
References
-
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/S0092-8674(02)01015-2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

