CD14 release induced by P2X7 receptor restricts inflammation and increases survival during sepsis
- PMID: 33135636
- PMCID: PMC7690950
- DOI: 10.7554/eLife.60849
CD14 release induced by P2X7 receptor restricts inflammation and increases survival during sepsis
Abstract
P2X7 receptor activation induces the release of different cellular proteins, such as CD14, a glycosylphosphatidylinositol (GPI)-anchored protein to the plasma membrane important for LPS signaling via TLR4. Circulating CD14 has been found at elevated levels in sepsis, but the exact mechanism of CD14 release in sepsis has not been established. Here, we show for first time that P2X7 receptor induces the release of CD14 in extracellular vesicles, resulting in a net reduction in macrophage plasma membrane CD14 that functionally affects LPS, but not monophosphoryl lipid A, pro-inflammatory cytokine production. Also, we found that during a murine model of sepsis, P2X7 receptor activity is important for maintaining elevated levels of CD14 in biological fluids and a decrease in its activity results in higher bacterial load and exacerbated organ damage, ultimately leading to premature deaths. Our data reveal that P2X7 is a key receptor for helping to clear sepsis because it maintains elevated concentrations of circulating CD14 during infection.
Keywords: CD14; LPS; cytokines; human; immunology; inflammation; mouse; purinergic signaling; sepsis.
Plain language summary
When the immune system detects an infection, it often launches an inflammatory response to fight off the disease. This defense mechanism is activated by a cascade of signaling molecules that can aggravate inflammation, causing it to damage the body’s own tissues and organs. This life-threatening reaction is referred to as sepsis, and kills around 11 million people each year. New approaches are therefore needed to help alleviate the damage caused by this condition. The inflammatory response is often triggered by proteins called receptors, which sit on the surface of immune cells. When these receptors are activated, they induce cells to secrete proteins that travel around the body and activate immune cells that can eliminate the infection. In 2016, a group of researchers showed that a receptor called P2X7 stimulates the release of a signaling molecule called CD14. Patients with sepsis often have elevated amounts of CD14 in their bloodstream. Yet, it remained unclear what causes this rise in CD14 and what role this molecule plays in the development of sepsis. Now, Alarcón-Vila et al. – including some of the researchers involved in the 2016 study – have investigated the role of P2X7 in mice undergoing sepsis. This was done by puncturing the mice’s intestines, causing bacteria to leak out and initiate an over-active immune response. Alarcón-Vila et al. found that mice lacking the P2X7 receptor had less CD14 and struggled to eliminate the bacterial infection from their system. This increase in bacteria caused excessive damage to the mice’s organs, ultimately leading to premature death. These findings suggest that P2X7 plays an important role in preventing the onset of sepsis by helping maintain high levels of CD14 following infection. This result could help to identify new therapies that reduce the mortality rates of septic infections.
© 2020, Alarcón-Vila et al.
Conflict of interest statement
CA, AB, Cd, CM, JM, HM, CG, PP No competing interests declared
Figures

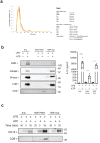
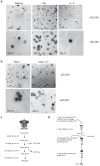
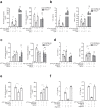

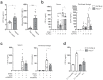

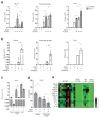

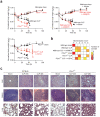


References
-
- Barratt-Due A, Pischke SE, Nilsson PH, Espevik T, Mollnes TE. Dual inhibition of complement and Toll-like receptors as a novel approach to treat inflammatory diseases-C3 or C5 emerge together with CD14 as promising targets. Journal of Leukocyte Biology. 2017;101:193–204. doi: 10.1189/jlb.3VMR0316-132R. - DOI - PMC - PubMed
-
- Baumann CL, Aspalter IM, Sharif O, Pichlmair A, Blüml S, Grebien F, Bruckner M, Pasierbek P, Aumayr K, Planyavsky M, Bennett KL, Colinge J, Knapp S, Superti-Furga G. CD14 is a coreceptor of Toll-like receptors 7 and 9. Journal of Experimental Medicine. 2010;207:2689–2701. doi: 10.1084/jem.20101111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

