Transition-Metal-Mediated Functionalization of White Phosphorus
- PMID: 33135828
- PMCID: PMC7894350
- DOI: 10.1002/chem.202001854
Transition-Metal-Mediated Functionalization of White Phosphorus
Abstract
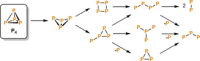
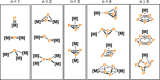
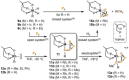
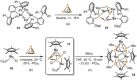
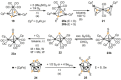
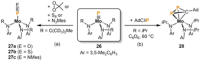
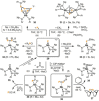
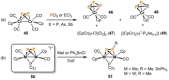
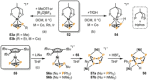
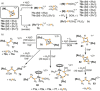
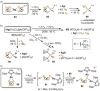
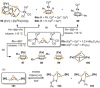
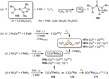
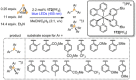
Recently there has been great interest in the reactivity of transition-metal (TM) centers towards white phosphorus (P4 ). This has ultimately been motivated by a desire to find TM-mediated alternatives to the current industrial routes used to transform P4 into myriad useful P-containing products, which are typically indirect, wasteful, and highly hazardous. Such a TM-mediated process can be divided into two steps: activation of P4 to generate a polyphosphorus complex TM-Pn , and subsequent functionalization of this complex to release the desired phosphorus-containing product. The former step has by now become well established, allowing the isolation of many different TM-Pn products. In contrast, productive functionalization of these complexes has proven extremely challenging and has been achieved only in a relative handful of cases. In this review we provide a comprehensive summary of successful TM-Pn functionalization reactions, where TM-Pn must be accessible by reaction of a TM precursor with P4 . We hope that this will provide a useful resource for continuing efforts that are working towards this highly challenging goal of modern synthetic chemistry.
Keywords: P ligands; coordination compounds; radicals; transition metals; white phosphorus.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





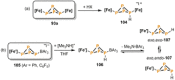
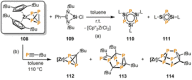
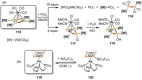
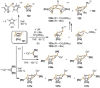




References
-
- Holleman A. F., Wiberg E., Wiberg N., Anorganische Chemie, Band 1 Grundlagen und Hauptgruppenelemente, de Gruyter, Berlin, 2017, p. 846.
-
- Peruzzini M., Gonsalvi L., Romerosa A., Chem. Soc. Rev. 2005, 34, 1038–1047. - PubMed
-
- Cossairt B. M., Piro N. A., Cummins C. C., Chem. Rev. 2010, 110, 4164–4177. - PubMed
-
- Corbridge D., Phosphorus: An Outline of its Chemistry, Biochemistry and Technology, Elsevier, New York, 1994.
-
- Elvers B., Ullmann F., Ullmann's encyclopedia of industrial chemistry, Wiley-VCH, Weinheim, 2011.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

