Live Imaging of Intracranial Lymphatics in the Zebrafish
- PMID: 33135960
- PMCID: PMC7790877
- DOI: 10.1161/CIRCRESAHA.120.317372
Live Imaging of Intracranial Lymphatics in the Zebrafish
Abstract
Rationale: The recent discovery of meningeal lymphatics in mammals is reshaping our understanding of fluid homeostasis and cellular waste management in the brain, but visualization and experimental analysis of these vessels is challenging in mammals. Although the optical clarity and experimental advantages of zebrafish have made this an essential model organism for studying lymphatic development, the existence of meningeal lymphatics has not yet been reported in this species.
Objective: Examine the intracranial space of larval, juvenile, and adult zebrafish to determine whether and where intracranial lymphatic vessels are present.
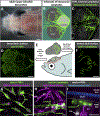
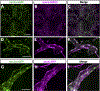
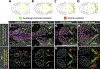
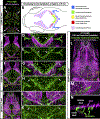
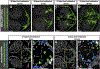
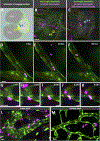
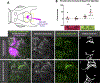
Methods and results: Using high-resolution optical imaging of the meninges in living animals, we show that zebrafish possess a meningeal lymphatic network comparable to that found in mammals. We confirm that this network is separate from the blood vascular network and that it drains interstitial fluid from the brain. We document the developmental origins and growth of these vessels into a distinct network separated from the external lymphatics. Finally, we show that these vessels contain immune cells and perform live imaging of immune cell trafficking and transmigration in meningeal lymphatics.
Conclusions: This discovery establishes the zebrafish as a important new model for experimental analysis of meningeal lymphatic development and opens up new avenues for probing meningeal lymphatic function in health and disease.
Keywords: brain; developmental biology; meninges; zebrafish.
Figures

Comment in
-
Lymphatics and the Brain: It's Time to Go Fishing.Circ Res. 2021 Jan 8;128(1):59-61. doi: 10.1161/CIRCRESAHA.120.318496. Epub 2021 Jan 7. Circ Res. 2021. PMID: 33411628 No abstract available.
References
-
- Ahn JH, Cho H, Kim JH, Kim SH, Ham JS, Park I, Suh SH, Hong SP, Song JH, Hong YK, et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature. 2019;572:62–66 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

