Developing a Standardized and Reusable Method to Link Distributed Health Plan Databases to the National Death Index: Methods Development Study Protocol
- PMID: 33136063
- PMCID: PMC7669437
- DOI: 10.2196/21811
Developing a Standardized and Reusable Method to Link Distributed Health Plan Databases to the National Death Index: Methods Development Study Protocol
Abstract
Background: Certain medications may increase the risk of death or death from specific causes (eg, sudden cardiac death), but these risks may not be identified in premarket randomized trials. Having the capacity to examine death in postmarket safety surveillance activities is important to the US Food and Drug Administration's (FDA) mission to protect public health. Distributed networks of electronic health plan databases used by the FDA to conduct multicenter research or medical product safety surveillance studies often do not systematically include death or cause-of-death information.
Objective: This study aims to develop reusable, generalizable methods for linking multiple health plan databases with the Centers for Disease Control and Prevention's National Death Index Plus (NDI+) data.
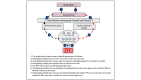
Methods: We will develop efficient administrative workflows to facilitate multicenter institutional review board (IRB) review and approval within a distributed network of 6 health plans. The study will create a distributed NDI+ linkage process that avoids sharing of identifiable patient information between health plans or with a central coordinating center. We will develop standardized criteria for selecting and retaining NDI+ matches and methods for harmonizing linked information across multiple health plans. We will test our processes within a use case comprising users and nonusers of antiarrhythmic medications.
Results: We will use the linked health plan and NDI+ data sets to estimate the incidences and incidence rates of mortality and specific causes of death within the study use case and compare the results with reported estimates. These comparisons provide an opportunity to assess the performance of the developed NDI+ linkage approach and lessons for future studies requiring NDI+ linkage in distributed database settings. This study is approved by the IRB at Harvard Pilgrim Health Care in Boston, MA. Results will be presented to the FDA at academic conferences and published in peer-reviewed journals.
Conclusions: This study will develop and test a reusable distributed NDI+ linkage approach with the goal of providing tested NDI+ linkage methods for use in future studies within distributed data networks. Having standardized and reusable methods for systematically obtaining death and cause-of-death information from NDI+ would enhance the FDA's ability to assess mortality-related safety questions in the postmarket, real-world setting.
International registered report identifier (irrid): DERR1-10.2196/21811.
Keywords: National Death Index; all-cause mortality; cause specific mortality; data linkage; distributed analysis; multisite research.
©Candace C Fuller, Wei Hua, Charles E Leonard, Andrew Mosholder, Ryan Carnahan, Sarah Dutcher, Katelyn King, Andrew B Petrone, Robert Rosofsky, Laura A Shockro, Jessica Young, Jea Young Min, Ingrid Binswanger, Denise Boudreau, Marie R Griffin, Margaret A Adgent, Jennifer Kuntz, Cheryl McMahill-Walraven, Pamala A Pawloski, Robert Ball, Sengwee Toh. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 02.11.2020.
Conflict of interest statement
Conflicts of Interest: CEL serves on the Executive Committee of the University of Pennsylvania's Center for Pharmacoepidemiology Research and Training. The Center receives funds for education from Pfizer and Sanofi. He recently received honoraria from the American College of Clinical Pharmacy Research Institute and the University of Florida College of Pharmacy. CEL's research is funded by the American Diabetes Association, Food and Drug Administration, and National Institutes of Health. CEL is a Special Government Employee of the Food and Drug Administration.
Figures

Similar articles
-
How do Orthopaedic Devices Change After Their Initial FDA Premarket Approval?Clin Orthop Relat Res. 2016 Apr;474(4):1053-68. doi: 10.1007/s11999-015-4634-x. Epub 2015 Nov 19. Clin Orthop Relat Res. 2016. PMID: 26584802 Free PMC article.
-
Validation of US CDC National Death Index mortality data, focusing on differences in race and ethnicity.BMJ Health Care Inform. 2023 Jul;30(1):e100737. doi: 10.1136/bmjhci-2023-100737. BMJ Health Care Inform. 2023. PMID: 37429673 Free PMC article.
-
Critical Care Network in the State of Qatar.Qatar Med J. 2019 Nov 7;2019(2):2. doi: 10.5339/qmj.2019.qccc.2. eCollection 2019. Qatar Med J. 2019. PMID: 31763205 Free PMC article.
-
A feasible method for linkage studies avoiding clerical review: linkage of the national HIV/AIDS surveillance databases with the National Death Index in Australia.Aust N Z J Public Health. 2007 Aug;31(4):308-12. doi: 10.1111/j.1753-6405.2007.00076.x. Aust N Z J Public Health. 2007. PMID: 17725006 Review.
-
Hyaluronidase (Vitrase)--ISTA: hyaluronidase--ISTA pharmaceuticals.Drugs R D. 2003;4(3):194-7. doi: 10.2165/00126839-200304030-00010. Drugs R D. 2003. PMID: 12757408 Review.
Cited by
-
Impact of site, size and severity of ischemic cerebrovascular stroke on sleep in a sample of Egyptian patients a polysomnographic study.BMC Neurol. 2023 Oct 26;23(1):387. doi: 10.1186/s12883-023-03438-6. BMC Neurol. 2023. PMID: 37884861 Free PMC article.
-
Folate intake, tissue levels, and colorectal cancer deaths in a national cohort established before folic acid fortification.Eur J Nutr. 2025 Jun 2;64(5):202. doi: 10.1007/s00394-025-03720-y. Eur J Nutr. 2025. PMID: 40455102
References
-
- Questions and Answers on FDA's Adverse Event Reporting System (FAERS) US Food and Drug Administration. 2018. [2018-03-28]. https://www.fda.gov/drugs/surveillance/questions-and-answers-fdas-advers....
-
- Colman E, Szarfman A, Wyeth J, Mosholder A, Jillapalli D, Levine J, Avigan M. An evaluation of a data mining signal for amyotrophic lateral sclerosis and statins detected in FDA's spontaneous adverse event reporting system. Pharmacoepidemiol Drug Saf. 2008 Nov;17(11):1068–76. doi: 10.1002/pds.1643. - DOI - PubMed
LinkOut - more resources
Full Text Sources

