High Glucose and Hypoxia-Mediated Damage to Human Brain Microvessel Endothelial Cells Induces an Altered, Pro-Inflammatory Phenotype in BV-2 Microglia In Vitro
- PMID: 33136275
- PMCID: PMC8942976
- DOI: 10.1007/s10571-020-00987-z
High Glucose and Hypoxia-Mediated Damage to Human Brain Microvessel Endothelial Cells Induces an Altered, Pro-Inflammatory Phenotype in BV-2 Microglia In Vitro
Abstract
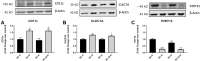
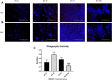
Diabetes is strongly linked to the development of Alzheimer's disease (AD), though the mechanisms for this enhanced risk are unclear. Because vascular inflammation is a consistent feature of both diabetes and AD, the cerebral microcirculation could be a key target for the effects of diabetes in the brain. The goal of this study is to explore whether brain endothelial cells, injured by diabetes-related insults, glucose and hypoxia, can affect inflammatory and activation processes in microglia in vitro. Human brain microvascular endothelial cells (HBMVECs) were either treated with 5 mM glucose (control), 30 mM glucose (high glucose), exposed to hypoxia, or exposed to hypoxia plus high glucose. HBMVEC-conditioned medium was then used to treat BV-2 microglia. Alterations in microglia phenotype were assessed through measurement of nitric oxide (NO), cytokine production, microglial activation state markers, and microglial phagocytosis. HBMVECs were injured by exposure to glucose and/or hypoxia, as assessed by release of LDH, interleukin (IL)-1β, and reactive oxygen species (ROS). HBMVECs injured by glucose and hypoxia induced increases in microglial production of NO, tumor necrosis factor-α (TNFα) and matrix metalloproteinase (MMP)-9. Injured HBMVECs significantly increased microglial expression of CD11c and CLEC7A, and decreased expression of the homeostatic marker P2RY12. Finally, bead uptake by BV-2 cells, an index of phagocytic ability, was elevated by conditioned media from injured HBMVECs. The demonstration that injury to brain endothelial cells by diabetic-associated insults, glucose and hypoxia, promotes microglial inflammation supports the idea that the cerebral microcirculation is a critical locus for the deleterious effects of diabetes in the AD brain.
Keywords: Alzheimer’s disease; Endothelial; Inflammation; Microglia; Vascular.
© 2020. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



Similar articles
-
Thrombin Signaling Contributes to High Glucose-Induced Injury of Human Brain Microvascular Endothelial Cells.J Alzheimers Dis. 2021;79(1):211-224. doi: 10.3233/JAD-200658. J Alzheimers Dis. 2021. PMID: 33252072
-
Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia.J Neuroinflammation. 2013 Feb 6;10:23. doi: 10.1186/1742-2094-10-23. J Neuroinflammation. 2013. PMID: 23388509 Free PMC article.
-
The microRNA miR-181c controls microglia-mediated neuronal apoptosis by suppressing tumor necrosis factor.J Neuroinflammation. 2012 Sep 6;9:211. doi: 10.1186/1742-2094-9-211. J Neuroinflammation. 2012. PMID: 22950459 Free PMC article.
-
A potential gliovascular mechanism for microglial activation: differential phenotypic switching of microglia by endothelium versus astrocytes.J Neuroinflammation. 2018 May 15;15(1):143. doi: 10.1186/s12974-018-1189-2. J Neuroinflammation. 2018. PMID: 29764475 Free PMC article.
-
Mechanisms of microglial activation in models of inflammation and hypoxia: Implications for chronic intermittent hypoxia.J Physiol. 2016 Mar 15;594(6):1563-77. doi: 10.1113/JP271502. J Physiol. 2016. PMID: 26890698 Free PMC article. Review.
Cited by
-
Graft ischemia post cell transplantation to the brain: Glucose deprivation as the primary driver of rapid cell death.Neurotherapeutics. 2025 Mar;22(2):e00518. doi: 10.1016/j.neurot.2024.e00518. Epub 2025 Jan 9. Neurotherapeutics. 2025. PMID: 39788838 Free PMC article.
-
Exosomal Mir-3613-3p derived from oxygen-glucose deprivation-treated brain microvascular endothelial cell promotes microglial M1 polarization.Cell Mol Biol Lett. 2023 Mar 5;28(1):18. doi: 10.1186/s11658-023-00432-1. Cell Mol Biol Lett. 2023. PMID: 36870962 Free PMC article.
-
NRF1-mediated microglial activation triggers high-altitude cerebral edema.J Mol Cell Biol. 2022 Sep 19;14(5):mjac036. doi: 10.1093/jmcb/mjac036. J Mol Cell Biol. 2022. PMID: 35704676 Free PMC article.
-
Chronic cerebral hypoperfusion induces venous dysfunction via EPAS1 regulation in mice.Nat Commun. 2025 Jul 8;16(1):6302. doi: 10.1038/s41467-025-61614-3. Nat Commun. 2025. PMID: 40628749 Free PMC article.
-
Argonaute-2 protects the neurovascular unit from damage caused by systemic inflammation.J Neuroinflammation. 2022 Jan 6;19(1):11. doi: 10.1186/s12974-021-02324-7. J Neuroinflammation. 2022. PMID: 34991639 Free PMC article.
References
-
- Blake R, Trounce IA (2014) Mitochondrial dysfunction and complications associated with diabetes. Biochim Biophys Acta 1840:1404–1412. 10.1016/j.bbagen.2013.11.007 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

