Gold-Catalyzed Synthesis of Small Rings
- PMID: 33136374
- PMCID: PMC8363095
- DOI: 10.1021/acs.chemrev.0c00697
Gold-Catalyzed Synthesis of Small Rings
Abstract
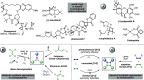
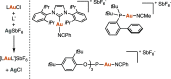
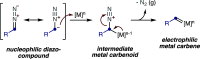
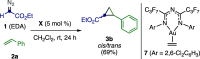
Three- and four-membered rings, widespread motifs in nature and medicinal chemistry, have fascinated chemists ever since their discovery. However, due to energetic considerations, small rings are often difficult to assemble. In this regard, homogeneous gold catalysis has emerged as a powerful tool to construct these highly strained carbocycles. This review aims to provide a comprehensive summary of all the major advances and discoveries made in the gold-catalyzed synthesis of cyclopropanes, cyclopropenes, cyclobutanes, cyclobutenes, and their corresponding heterocyclic or heterosubstituted analogs.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




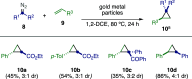
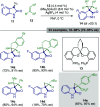

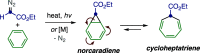
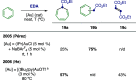




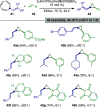
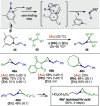
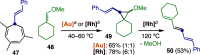
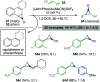















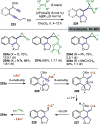
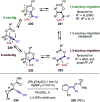
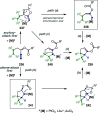
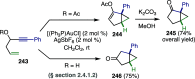







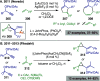

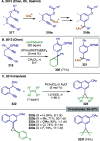
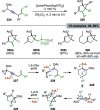

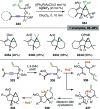













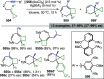
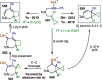

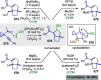

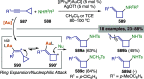
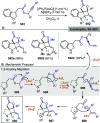
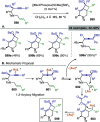

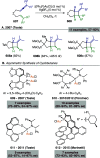

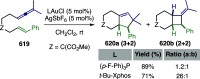





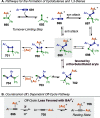
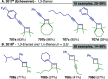
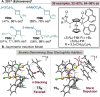

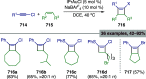
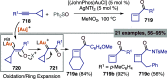
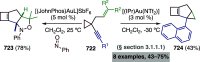
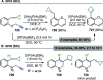
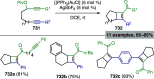



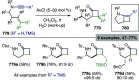
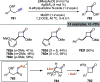
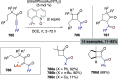
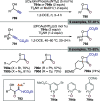
References
-
- Freund A. Ueber Trimethylen. J. Prakt. Chem. 1882, 26, 367–377. 10.1002/prac.18820260125. - DOI
-
- Willstätter R.; Bruce J. Zur Kenntnis der Cyclobutanreihe. Ber. Dtsch. Chem. Ges. 1907, 40, 3979–3999. 10.1002/cber.19070400407. - DOI
-
- Strained Organic Molecules; Greenberg A.; Liebman J. F., Eds.; Academic Press: New York, 1978.
Publication types
LinkOut - more resources
Full Text Sources

