Supporting Teen Problem-Solving (STEPS) 3 year outcomes: Preventing diabetes-specific emotional distress and depressive symptoms in adolescents with type 1 diabetes
- PMID: 33136423
- PMCID: PMC8279264
- DOI: 10.1037/ccp0000608
Supporting Teen Problem-Solving (STEPS) 3 year outcomes: Preventing diabetes-specific emotional distress and depressive symptoms in adolescents with type 1 diabetes
Abstract
Objective: This article reports the 3-year outcomes for the Supporting Teen Problem-Solving (STePS) multisite Randomized Control Trial (RCT); reporting the overall impact of the STePS trial, and the differential impact of each arm of the trial (a resilience promoting intervention [PRP T1D] vs. a diabetes education intervention [EI]) on diabetes-specific emotional distress and depressive symptoms.
Method: Participants included 264 adolescents with Type 1 diabetes (T1D), ages 14-18, in Chicago and San Francisco. Both intervention arms lasted 4.5 months and assessments were conducted at baseline, postintervention (4.5 months), and 5 follow-up visits (8, 12, 16, 28, and 40 months from baseline). Intervention efficacy was investigated using latent growth curve modeling (LGCM) to analyze the rate and shape of change of outcomes from preintervention across postintervention and follow-up time points.
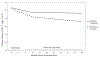
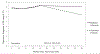
Results: Mean age of participants was 15.7 years, mean T1D duration was 6.9 years, mean HbA1c at baseline was 9.1%. The sample was diverse with nearly 35% identifying as racial or ethnic minorities, and 60% were female. PRP T1D participants reported significantly lower diabetes distress compared with EI participants, and the effect size increased over time. For the pooled sample, while 40% of youth reported elevated distress at baseline, only 23% reported elevated distress 3 years postintervention. Moreover, PRP T1D participants experienced a significant decline in depressive symptoms from 16 to 40 months postbaseline, while participants in the education arm did not.
Conclusions: Results from the 3-year outcomes assessment demonstrate the robust effects of PRP T1D in adolescents with declines in distress and depressive symptoms. (PsycInfo Database Record (c) 2020 APA, all rights reserved).
Figures


References
-
- Abualula N, Rodan M, Milligan R, Jacobsen K (2018). Self-rated health among American adolescents with type 1 diabetes in the T1D exchange clinic registry. Journal of Diabetes and its Complications, 32, 83–88. - PubMed
-
- Akaike H (1987). Factor analysis and AIC. Psychometrika, 52(3), 317–332.
-
- Bollen KA, & Curran PJ (2006). Latent curve models: A structural equation perspective. Hoboken, N.J.: Wiley.
-
- Browne MW, & Cudeck R (1992). Alternative ways of assessing model fit. Sociological Methods & Research, 21(2), 230–258.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

